Text Solution
Verified by Experts
Topper's Solved these Questions
ALDEHYDES, KETONES AND CARBOXYLIC ACIDS
PRADEEP|Exercise HIGHER ORDER THINKING SKILLS (HOTS PROBLEMS)|5 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
PRADEEP|Exercise VALUE BASED QUESTIONS WITH ANSWERS|3 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
PRADEEP|Exercise ADDITIONAL QUESTIONS (LONG ANSWER QUESTIONS)|10 VideosALCOHOLS, PHENOLS AND ETHERS
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATIONS.|29 VideosAPPENDIX
PRADEEP|Exercise MODEL TEST PAPER <br> (Section C )|15 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-ALDEHYDES, KETONES AND CARBOXYLIC ACIDS -HIGHER ORDER THINKING SKILLS (QUESTIONS AND PROBLEMS WITH ANSWER/SOLUTION)
- Accout for the following : (i) Oxidation of toluence to C(6)H(5)CHO wi...
Text Solution
|
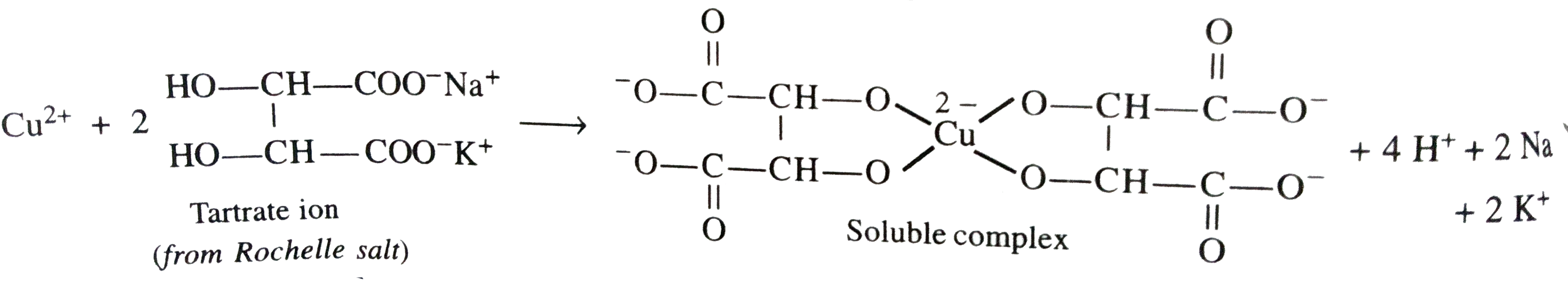
- What is the function of Rochelle salt in Fehling's solution ?
Text Solution
|
- Addition of Grignard reagents to dry ice followed by hydrolysis gives ...
Text Solution
|
- Assertion : Alkyl benzene is not prepared by Friedel Craft alkylation ...
Text Solution
|
- Tert-Butylbenzene does not benzoic acid on oxidation with acidic KMnO(...
Text Solution
|
- Explain why the carbonyl oxygen atom of a carboxylic acid is more basi...
Text Solution
|
- Me(3)C CH(2)COOH is more acidic than Me(3)SiCH(2)COOH.
Text Solution
|
- Identify compounds (A-D) in the following reactions: <img src="https...
Text Solution
|
- Suggest appropriate structures for the missing compound. (The number o...
Text Solution
|
- Write the intermediate steps for the following reaction. C(6)H(5)CH(...
Text Solution
|
- Identify A,B and C are give their structures.
Text Solution
|
- Arrange the following in decreasing ease of acid-catalysed esterificat...
Text Solution
|
 .
.