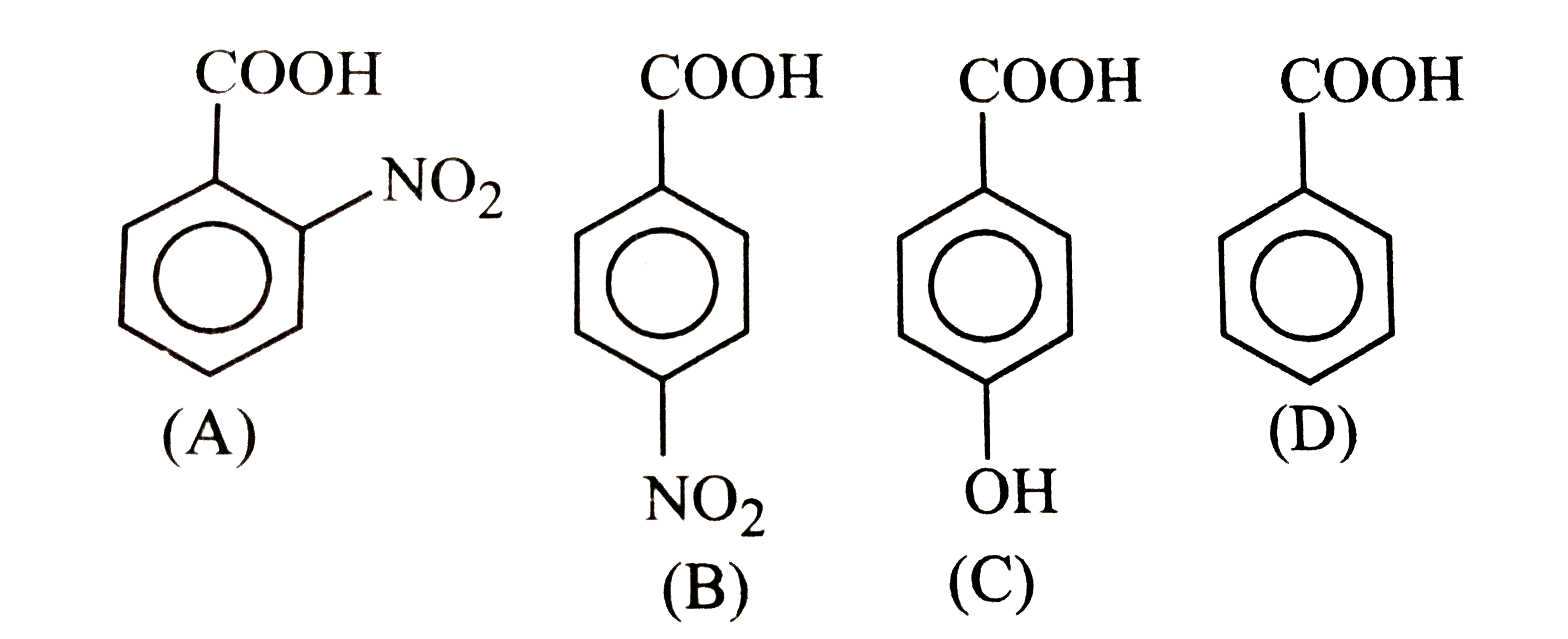
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALDEHYDES, KETONES AND CARBOXYLIC ACIDS
PRADEEP|Exercise COMPETITION FOCUS JEE (MAIN AND ADVANCED)/MEDICAL ENTRANCE SPECIEAL (MULTIPLE CHOICE QUESTIONS-II WITH ONE OR MORE THAN ONE CORRECT ANSWER)|1 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
PRADEEP|Exercise COMPETITION FOCUS JEE (MAIN AND ADVANCED)/MEDICAL ENTRANCE SPECIEAL (MULTIPLE CHOICE QUESTIONS-II WITH ONE CORRECT ANSWER)|12 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
PRADEEP|Exercise VALUE BASED QUESTIONS WITH ANSWERS|3 VideosALCOHOLS, PHENOLS AND ETHERS
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATIONS.|29 VideosAPPENDIX
PRADEEP|Exercise MODEL TEST PAPER <br> (Section C )|15 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-ALDEHYDES, KETONES AND CARBOXYLIC ACIDS -COMPETITION FOCUS JEE (MAIN AND ADVANCED)/MEDICAL ENTRANCE SPECIEAL (MULTIPLE CHOICE QUESTIONS-I WITH ONE CORRECT ANSWER)
- Among the following compounds the most acidic is
Text Solution
|
- The correct order of acidity for following compounds is
Text Solution
|
- Arrange the following acids in order of their increasing acidity
Text Solution
|
- The correct increasing order of the acid strength of benzoic acid (I),...
Text Solution
|
- Among the given compounds, the most susceptible to nucleophilic attack...
Text Solution
|
- Compound (A) ,C(8)H(9)Br gives a white precipitate when warmed with a...
Text Solution
|
- When acetyl chloride reacts with sodium propionate, the product formed...
Text Solution
|
- The correct set of reagents for the following conversions is
Text Solution
|
- Among the following compounds, the one (s) that gives (gives) efferves...
Text Solution
|
- A liquid was mixed with ethanol and a drop of concentrated H(2) SO(4) ...
Text Solution
|
- Sodium ethoxide has reacted with ethanoyl chloride. The compound that ...
Text Solution
|
- CH(3)COOH overset(LiAlH(4))toA A+CH(3)COOH overset(H(3)O^(+))to B+H(...
Text Solution
|
- Methyl benzoate can prepared by
Text Solution
|
- CH(3)-CH(2)-overset(O)overset(||)(C )-OC(2)H(5) overset(Naoverset(**)(...
Text Solution
|
- Consider the following compounds: The correct decreasing order of...
Text Solution
|
- Which of the the following esters gets hydrolysed most easily under al...
Text Solution
|
- When CH(2)=CH -CO OH is reduced with LiAlH(4) the compound obtained wi...
Text Solution
|
- Propionic acid with Br(2)//P yields a dibromoproduct. Its structure wo...
Text Solution
|
- A compound undergoes the following sequence of reactions: C(3)H(5)N ...
Text Solution
|
- Which of the following acids on heating loses a molecul of H(2)O to fo...
Text Solution
|