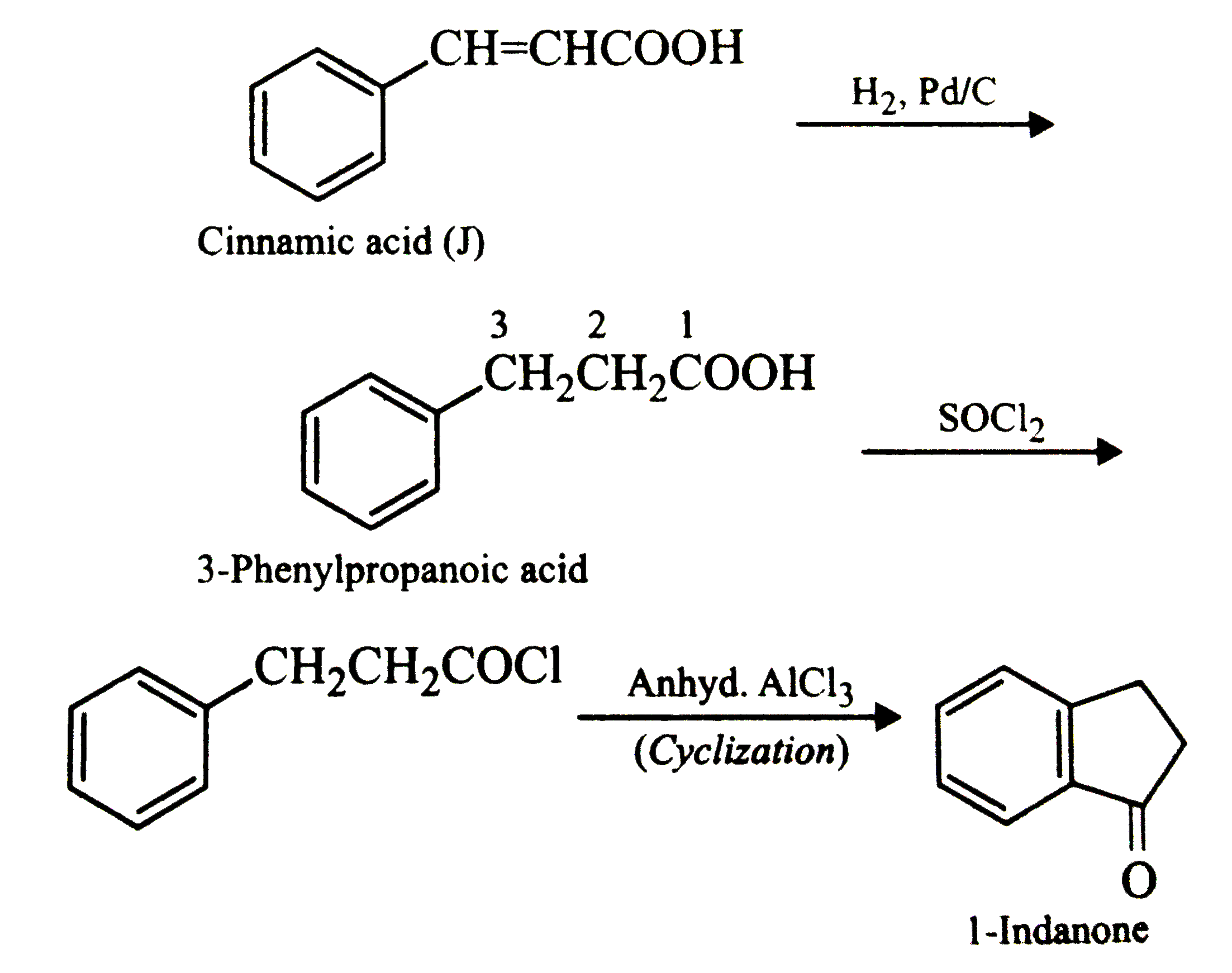Text Solution
Verified by Experts
Topper's Solved these Questions
ALDEHYDES, KETONES AND CARBOXYLIC ACIDS
PRADEEP|Exercise COMPETITION FOCUS JEE (MAIN AND ADVANCED)/MEDICAL ENTRANCE SPECIEAL (V. MATRIX-MATCH TYPE QUESTIONS)|1 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
PRADEEP|Exercise COMPETITION FOCUS JEE (MAIN AND ADVANCED)/MEDICAL ENTRANCE SPECIEAL (VI. INTEGER TYPE QUESIONS)|11 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
PRADEEP|Exercise COMPETITION FOCUS JEE (MAIN AND ADVANCED)/MEDICAL ENTRANCE SPECIEAL (MULTIPLE CHOICE QUESTIONS-II WITH ONE CORRECT ANSWER)|12 VideosALCOHOLS, PHENOLS AND ETHERS
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATIONS.|29 VideosAPPENDIX
PRADEEP|Exercise MODEL TEST PAPER <br> (Section C )|15 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-ALDEHYDES, KETONES AND CARBOXYLIC ACIDS -COMPETITION FOCUS JEE (MAIN AND ADVANCED)/MEDICAL ENTRANCE SPECIEAL (MULTIPLE CHOICE QUESTIONS COMPREHENSION TYPE)
- In chemical analysis, the presence of a methyl ketonic group is tested...
Text Solution
|
- In chemical analysis, the presence of a methyl ketonic group is tested...
Text Solution
|
- The acidic strength of saturated aliphatic carboxylic acids depends ma...
Text Solution
|
- The acidity of carboxylic acid is determined by the nature of the alky...
Text Solution
|
- A tertiary alcohol H upon acid catalysed dehydration gives a product I...
Text Solution
|
- A tertiary alcohol H upon acid catalysed dehydration gives a product I...
Text Solution
|
- A tertiary alcohol H upon acid catalysed dehydration gives a product I...
Text Solution
|
- In the following reaction sequence, the compound J is an interemedia...
Text Solution
|
- In the following reaction sequence, the compound J is an interemedia...
Text Solution
|
- P and Q are isomers of dicarboxylic acid C(4)H(4)O(4). Both decolouriz...
Text Solution
|
- P and Q are isomers of dicarboxylic acid C(4)H(4)O(4). Both decolouriz...
Text Solution
|