(i) `C_(6)H_(5)NH_(2)`, N is directly linked to the benzene ring and hence the lone pair of electrons on the N-atom is delocalized over the benzene ring. In contrast, N in `C_(6)H_(5)CH_(2)NH_(2)` is not directly linked to the benzene ring and hence its lone pair is not delocalized over the benzene ring. in other words, the lone pair of electrons on the N-atom in `C_(6)H_(5)CH_(2)NH_(2)` is more easily available for protonation than that on the N-atom in `C_(6)H_(5)NH_(2)`. thus, `C_(6)H_(5)CH_(2)NH_(2)` is more basic than `C_(6)H_(5)NH_(2)`. Now due to -I-effect of the `C_(6)H_(5)` group, electron density on the N-atom in `C_(6)H_(5)CH_(2)NH_(2)` is lower than that on the N-atom in `C_(2)H_(5)NH_(2)`, therefore, `C_(2)H_(5)NH_(2)` is more basic than `C_(6)H_(5)CH_(2)NH_(2)`. Lowerver, both the them are stronger bases than `NH_(3)`. further due to +I-effect of the two `C_(2)H_(5)` `C_(2)H_(5)` groups in `(C_(2)H_(5))_(2)NH` as compared to one in `C_(2)H_(5)NH_(2),(C_(2)H_(5))_(2)NH` is a stronger base than `C_(2)H_(5)NH_(2)`. thus, the overall basic strength increases in the order:
`C_(6)H_(5)NH_(2) lt NH_(3) lt C_(6)H_(5)CH_(2)NH_(2) lt C_(2)H_(5)NH_(2) lt (C_(2)H_(5))_(2)NH`
(ii) In `C_(2)H_(5)NH_(2),(C_(2)H_(5))_(2)NH and (C_(2)H_(5))_(3)N`, the +I-effect of the `C_(2)H_(5)` group/s increases the electron density on the N-atom. however, in `C_(6)H_(5)NH_(2)`, the electron density on the N-atom decreases due to delocalization of the lone pair of electrons over the benzene ring. therefore, all the three ethylamines are more basic than `C_(6)H_(5)NH_(2)`.
The relative basic strength of `C_(2)H_(5)NH_(2),(C_(2)H_(5))_(2)NH and (C_(2)H_(5))_(3)N` depends upon the stabilization of their corresponding conjugate acids (formed as a result of accepting a proton from water) by a number of factors such as H-bonding, steric hindrance of the alkyl groups and +I-effect of the alkyl groups. All of factors such as H-bonding, steric hindrance of the alkyl groups and +I-effect of the alkyl groups. all these factors are favourable for `2^(@)` amines, therefore, `(C_(2)H_(5))_(2)NH` is a stronger base than `C_(2)H_(5)NH_(2) and (C_(2)H_(5))_(3)N`. since `C_(2)H_(5)` group is bigger, it exerts some steric hindrance to H-bonding. therefore, stabilization of the conjugate acid derived from `(C_(2)H_(5))_(3)N` due to +I-effect is greater than the stabilization of the conjugate acid derived from `C_(2)H_(5)NH_(2)` by H-bonding i.e.,

Therefore, `(C_(2)H_(5))_(3)N` is more basic than `C_(2)H_(5)NH_(2)`. The overall basic strength of the four amines increases in the order: `C_(6)H_(5)NH_(2) lt C_(2)H_(5)NH_(2) lt (C_(2)H_(5))_(3) lt (C_(2)H_(5))_(2) NH`
(iii) As explained in answer (i) above, `C_(6)H_(5)CH_(2)NH_(2)` is more basic than `C_(6)H_(5)NH_(2)`. now due to +I-effect of the `CH_(3) ` groups , th electron density on the N-atom in `CH_(3)NH_(2),(CH_(3))_(2)NH and (CH_(3))_(3)N` increases. however, in `C_(6)H_(5)NH_(2) and C_(6)H_(5)CH_(2)NH_(2)` electron density on the N-atom decreases due to electron-withdrawig resonance effect (i.e., -R-effect) of the `C_(6)H_(5)` group in `C_(6)H_(5)CH_(2)NH_(2)`. therefore, all the three methylamines are more basic than `C_(6)H_(5)NH_(2) and C_(6)H_(5)CH_(2)NH_(2)`.
now, the relative basic strength of `CH_(3)NH_(2),(CH_(3))_(2)NH` and `(CH_(3))_(3)N` depends upon the stabilization of their conjugate acids (formed as a result of accepting a proton from `H_(2)O`) by a number of factors such as H-bonding, steric hindrance of the alkyl groups and -I-effect of the alkyl groups. all these such as H-bonding, steric hindrance of the alkyl groups and +I-effect of the alkyl groups. all these factors are favourable for `2^(@)` amines, therefore, `(CH_(3))_(2)NH` is a stronger base than `CH_(3)NH_(2) and (CH_(3))_(3)N`. since `CH_(3) ` group is the smallest, it does not exert any steric hindrance. therefore, stabilization of the conjugate acid derived from `CH_(3)NH_(2)` due to H-bonding is greater than that of the conjugate acid derived from `(CH_(3))_(3)N` due to +I-effect of the three `CH_(3)` groups, i.e.,
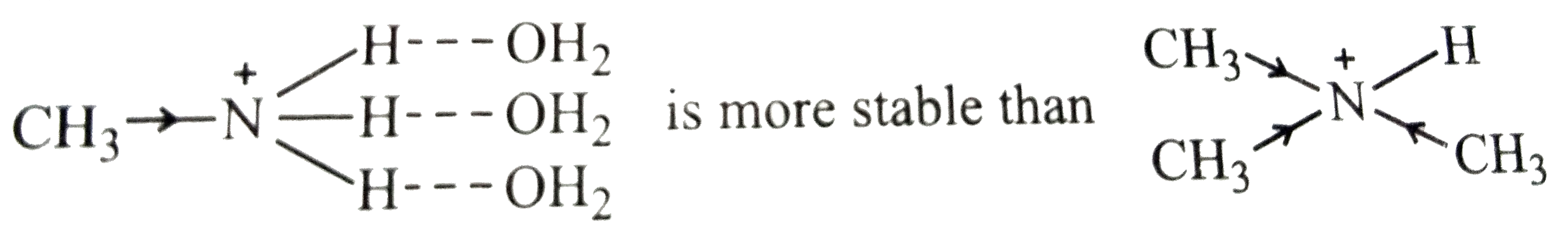
.
Therefore, `CH_(3)NH_(2)` is a stronger base than `(CH_(3))_(3)N`. thus, the overall basic strength of the five amines increases in the order:
`C_(6)H_(5)NH_(2) lt C_(6)H_(5) CH_(2)NH_(2) lt (CH_(3))_(3) N lt CH_(3)NH_(2) lt (CH_(3))_(2)NH`.

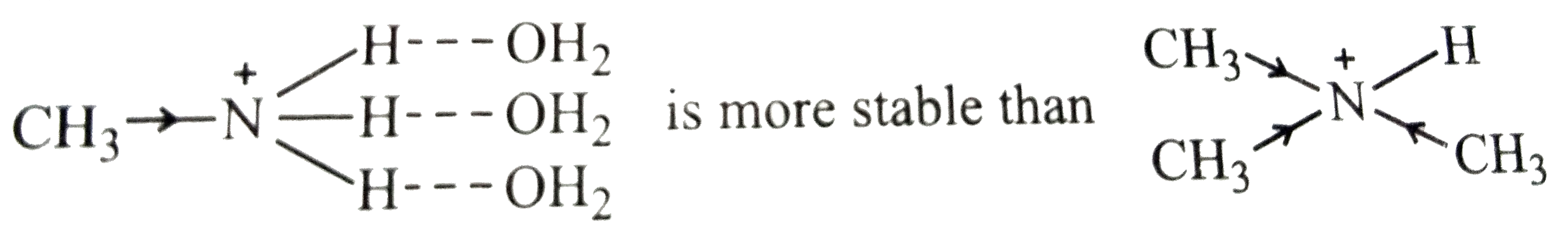 .
.