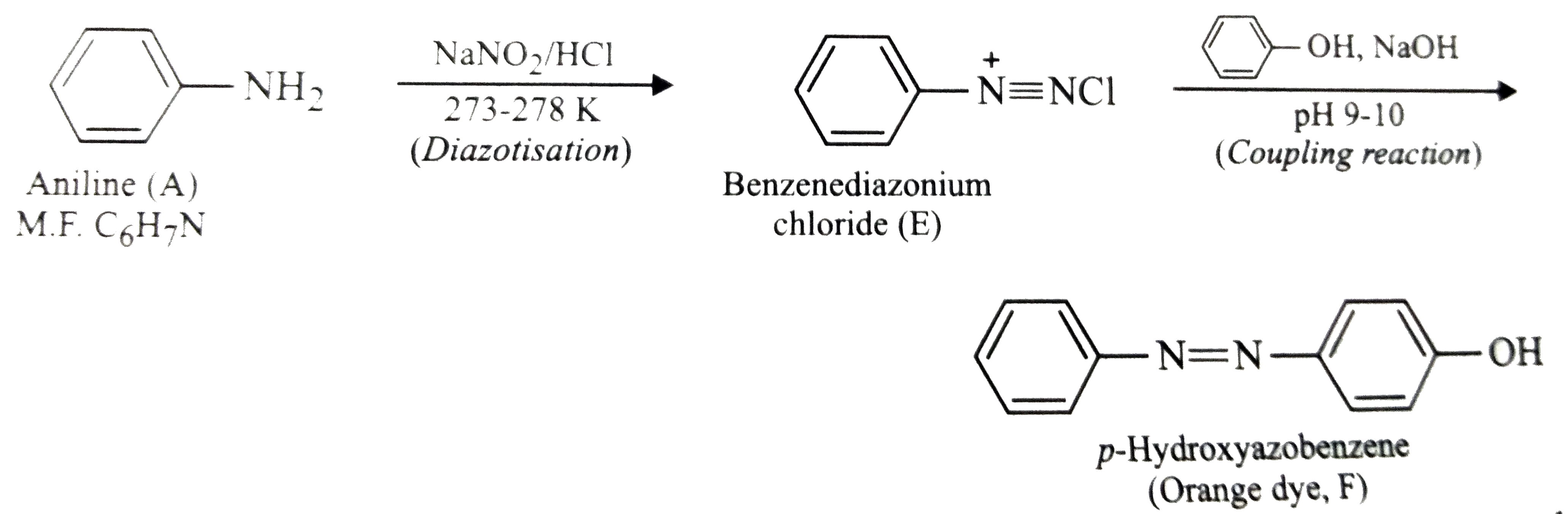
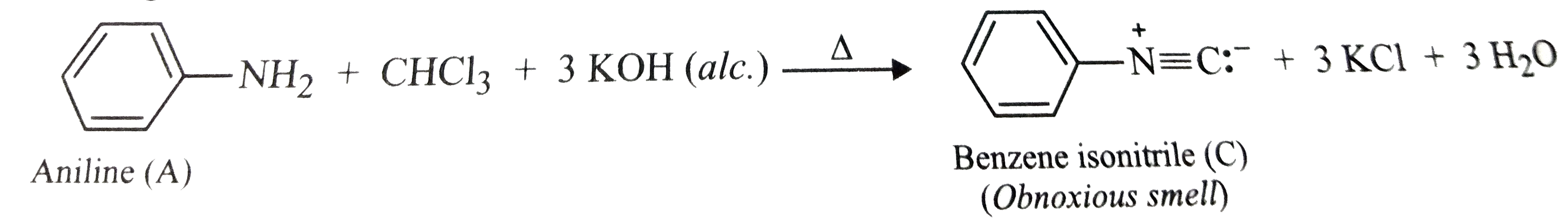
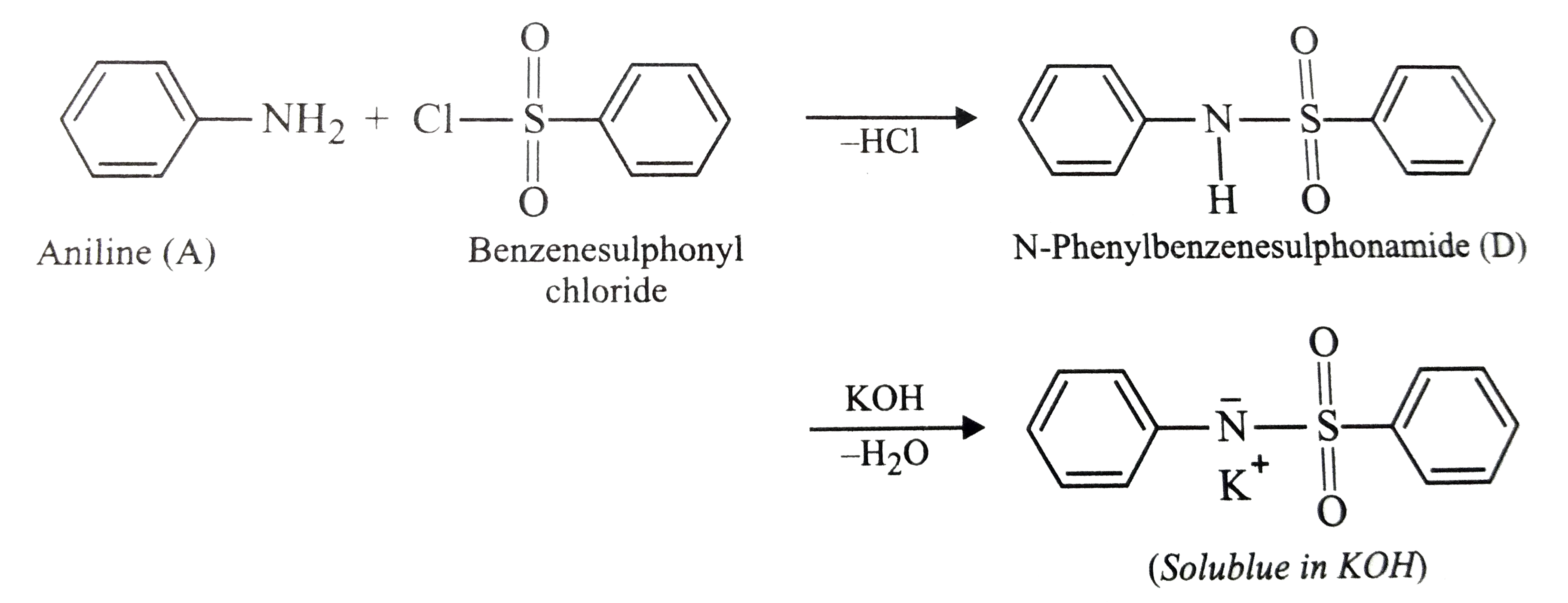
Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING NITROGEN
PRADEEP|Exercise ADDITIONAL QUESTIONS (VERY SHORT ANSWER QUESTIONS)|59 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
PRADEEP|Exercise ADDITIONAL QUESTIONS (SHORT ANSWER QUESTIONS)|79 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (ASSERTION AND REASON TYPE QUESTIONS)|7 VideosHALOALKANES AND HALOARENES
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATION|22 VideosP-BLOCK ELEMENTS
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATION|25 Videos
Similar Questions
Explore conceptually related problems