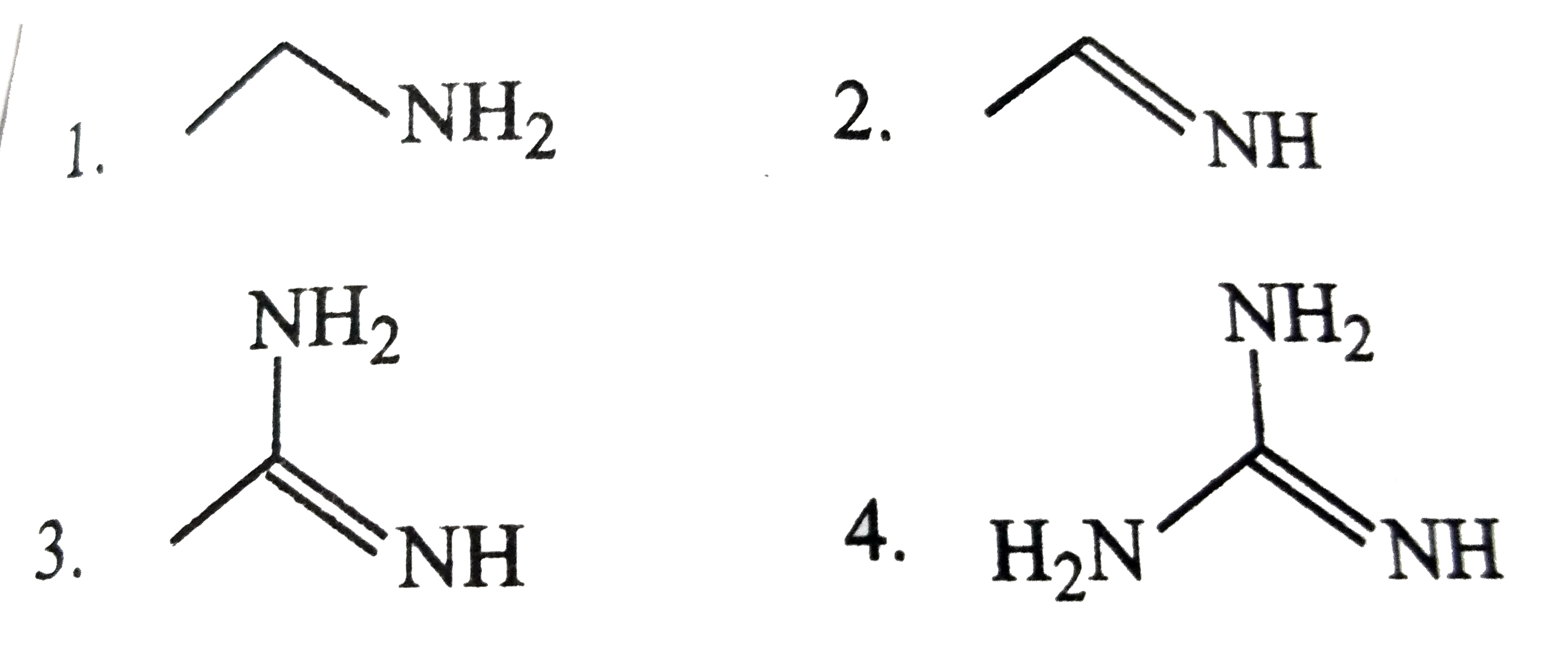
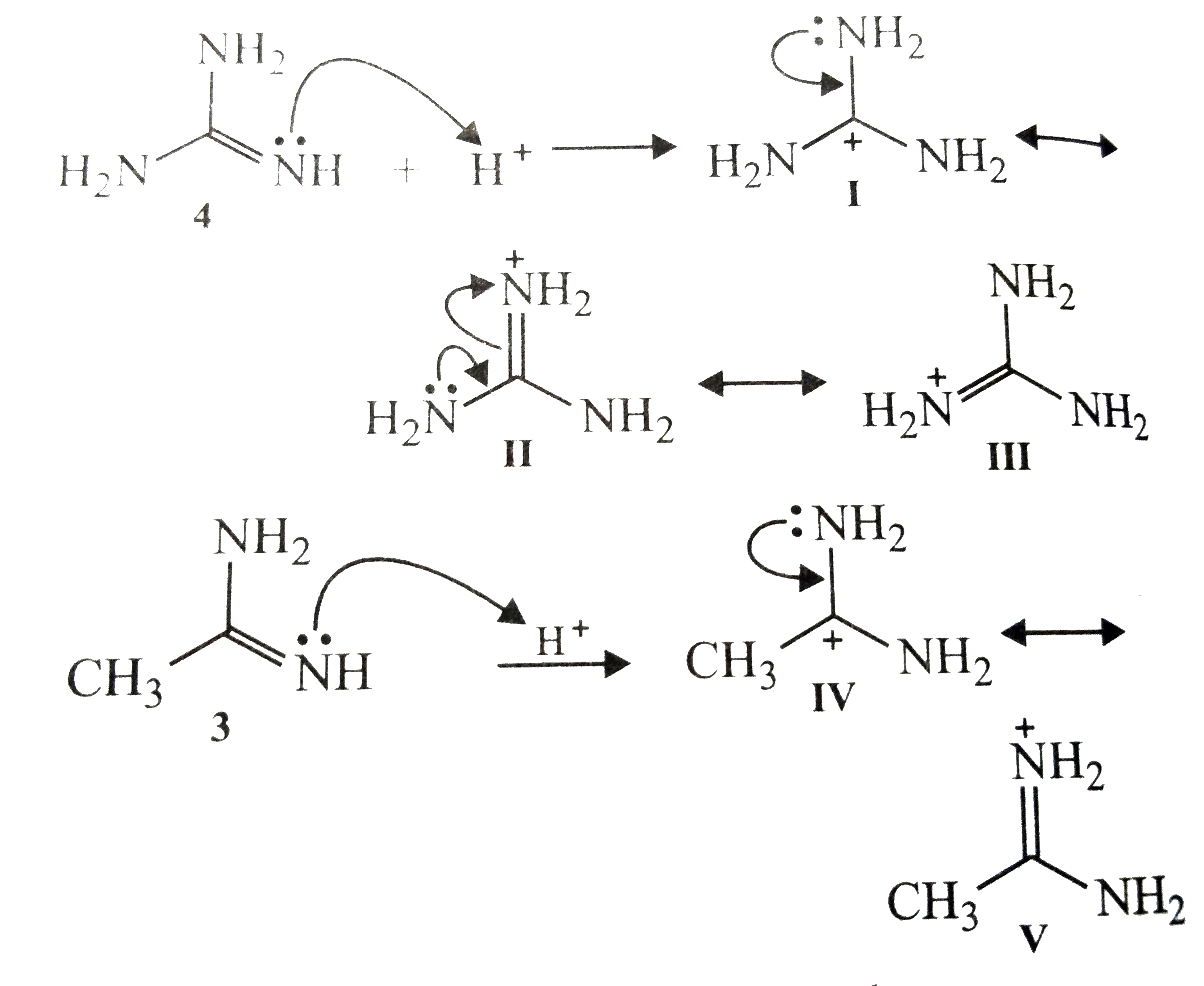
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING NITROGEN
PRADEEP|Exercise COMPETITION FOCUS JEE (MAIN AND ADVANCED)/MEDICAL ENTRANCE SPECIAL (MULTIPLE CHOICE QUESTIONS-II)|5 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
PRADEEP|Exercise COMPETITION FOCUS JEE (MAIN AND ADVANCED)/MEDICAL ENTRANCE SPECIAL (MULTIPLE CHOICE QUESTIONS-III COMPREHENSION TYPE)|9 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
PRADEEP|Exercise VALUE BASED QUESTIONS WITH ANSWERS|3 VideosHALOALKANES AND HALOARENES
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATION|22 VideosP-BLOCK ELEMENTS
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATION|25 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-ORGANIC COMPOUNDS CONTAINING NITROGEN-COMPETITION FOCUS JEE (MAIN AND ADVANCED)/MEDICAL ENTRANCE SPECIAL (MULTIPLE CHOICE QUESTIONS-I)
- Among the following dissocitation constant is highest for .
Text Solution
|
- In pyrrole the electron density is maximum on
Text Solution
|
- The correct order of basicity of the following
Text Solution
|
- The correct order of basicities of the following compounds is
Text Solution
|
- Strongest base is
Text Solution
|
- Order of the basic strength of the following compounds is
Text Solution
|
- A compound with molecular mass 180 is acylated with CH(3)COCl to get a...
Text Solution
|
- In the reaction shown below, the major product formed is/are
Text Solution
|
- The product formed by the reaction of an aldehyde with a primary amine...
Text Solution
|
- Reaction of cyclohexanone with dimethylamine in the presence of cataly...
Text Solution
|
- The major organic product formed from the following reaction
Text Solution
|
- In the following reactions, the product S is
Text Solution
|
- On heating an aliphatic primary amine with chloroform and enthanolic p...
Text Solution
|
- A positive carbylamine test is given by:
Text Solution
|
- The gas leaked from a storage tank of the Union Carbide plant in Bhopa...
Text Solution
|
- An organic compound (C3H9N) (A) when treated with nitrous acid , gave ...
Text Solution
|
- An organic compound 'A' on reduction give compound 'B' which on reacti...
Text Solution
|
- The optically active compound (X) on treatment with NaNO(2)//HCl ...
Text Solution
|
Text Solution
|
- Treatment of cyclobutylmethyamine with nitrous acid does not give .
Text Solution
|