Text Solution
Verified by Experts
Topper's Solved these Questions
BIOMOLECULES
PRADEEP|Exercise MATCHING TYPE QUESTIONS|2 VideosBIOMOLECULES
PRADEEP|Exercise ASSERTION AND REASON TYPE QUESTIONS|7 VideosBIOMOLECULES
PRADEEP|Exercise NCERT (EXEMPLAR PROBLEMS) (With answers, Hints And Solution) (Multiple Choice Questions-II )|9 VideosAPPENDIX
PRADEEP|Exercise MODEL TEST PAPER <br> (Section C )|15 VideosCHEMICAL KINETICS
PRADEEP|Exercise ADVANCED PROBLEMS FOR COMPETITIONS|14 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-BIOMOLECULES -Short Answer Questions
- Sucrose is dextrorotatory but the mixture obtained after hydrolysis is...
Text Solution
|
- Amino acids behave like salts rather than simple amines or carboxylic ...
Text Solution
|
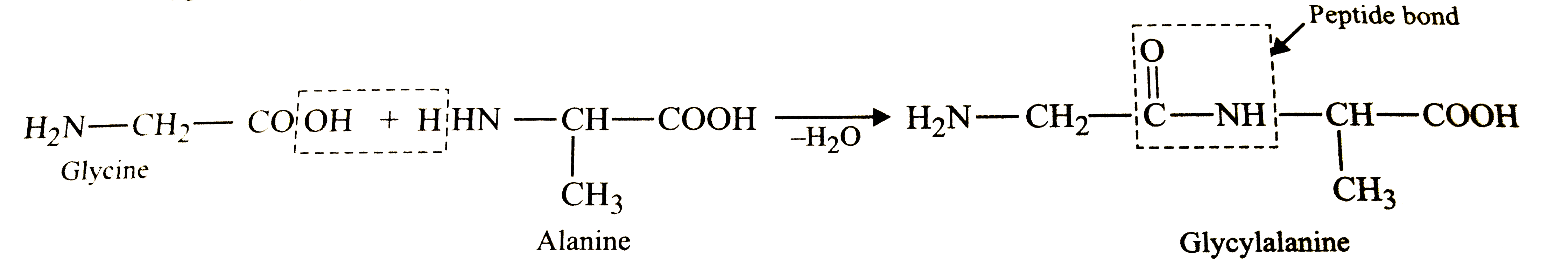
- Structure of glycine and alanine are given below. Show the peptide lin...
Text Solution
|
- Protein found in a biological system with a unique three-dimensional s...
Text Solution
|
- Activation energy for the acid catalysed hydrolysis of sucrose is 6.22...
Text Solution
|
- How do you explain the presence of an aldehydic group in a glucose mol...
Text Solution
|
- Which moieties of nucleosides are involved in the formation of phospho...
Text Solution
|
- What are glycosidic linkages ? In which type of biomolecules, are they...
Text Solution
|
- Which monosaccharide units are present in strach, cellulose and glucos...
Text Solution
|
- How do enzymes help a substrate to be attacked by the reagent effectiv...
Text Solution
|
- Descrive the term D- and L-configuration used for amino acids with exm...
Text Solution
|
- How will you distinguish 1^(@) and 2^(@) hydroxyl groups present in gl...
Text Solution
|
- Coagulation of egg white on boiling is an example of denaturation of p...
Text Solution
|
- Write the major classes in which the carbohydrates are divided dependi...
Text Solution
|
- Carbohydrates are classified into monosaccharides , oligosaccharides a...
Text Solution
|
- What are reducing sugars. Give an example each of a reducing sugar and...
Text Solution
|
- Write chemical reaction for following conversions glucose into gluco...
Text Solution
|
- What happenes when D-glucose is treated with the following reagents? ...
Text Solution
|
- How will you obtain from glucose : (i) gluconic acid (ii) n-hexane ?
Text Solution
|
- Give chemical reactions to show glucose : (a) all the six carbon ato...
Text Solution
|