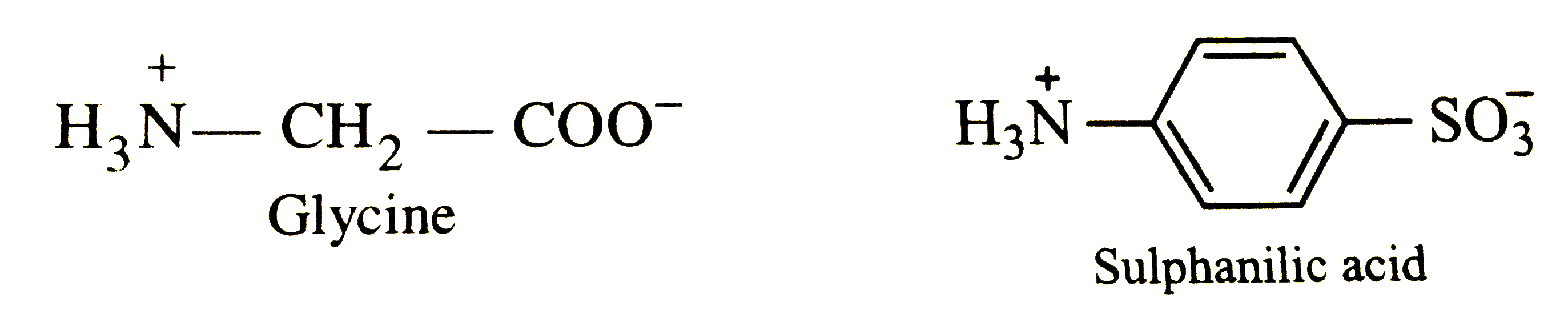Text Solution
Verified by Experts
Topper's Solved these Questions
BIOMOLECULES
PRADEEP|Exercise Higher order Thinking skills (Questions and problems with answers/solution(Hots Questions)|9 VideosBIOMOLECULES
PRADEEP|Exercise Higher order Thinking skills (Hots Problems )|1 VideosBIOMOLECULES
PRADEEP|Exercise LONG ANSWER QUESTIONS|11 VideosAPPENDIX
PRADEEP|Exercise MODEL TEST PAPER <br> (Section C )|15 VideosCHEMICAL KINETICS
PRADEEP|Exercise ADVANCED PROBLEMS FOR COMPETITIONS|14 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-BIOMOLECULES -ADDITIONAL QUESTIONS (Very Short Answer Questions)
- In what respect would any two naturally occuring amino acids differ fr...
Text Solution
|
- What are zwitterions ?Give one example.
Text Solution
|
- Write the zwitterion structure for glycine.
Text Solution
|
- Isoelectric point is a
Text Solution
|
- (a) Give one example each of essential and non-essential amino acids. ...
Text Solution
|
- What are the ultimate products of the digestion of proteins ?
Text Solution
|
- What is a prothetic group ?
Text Solution
|
- Write the name of linkage joining two amino acids.
Text Solution
|
- What is a peptide bond?
Text Solution
|
- What is a polypeptide ? Give one example.
Text Solution
|
- What type of bonding occurs in globular protein?
Text Solution
|
- What forces are responsible for stability of alpha-helix ?
Text Solution
|
- Name the central atom present in haemoglobin and chlorophyll.
Text Solution
|
- Give one example each for (a) Fibrous Protein (b) Globular Protein
Text Solution
|
- Differentiate between Keratin and insulin.
Text Solution
|
- What causes sickle cell anaemia?
Text Solution
|
- When protein is subjected to denaturation
Text Solution
|
- Give the biological functions of the following proteins: (i) Haemog
Text Solution
|
- What are coenzymes ? Give example.
Text Solution
|
- Name the enzyme present in human saliva.
Text Solution
|