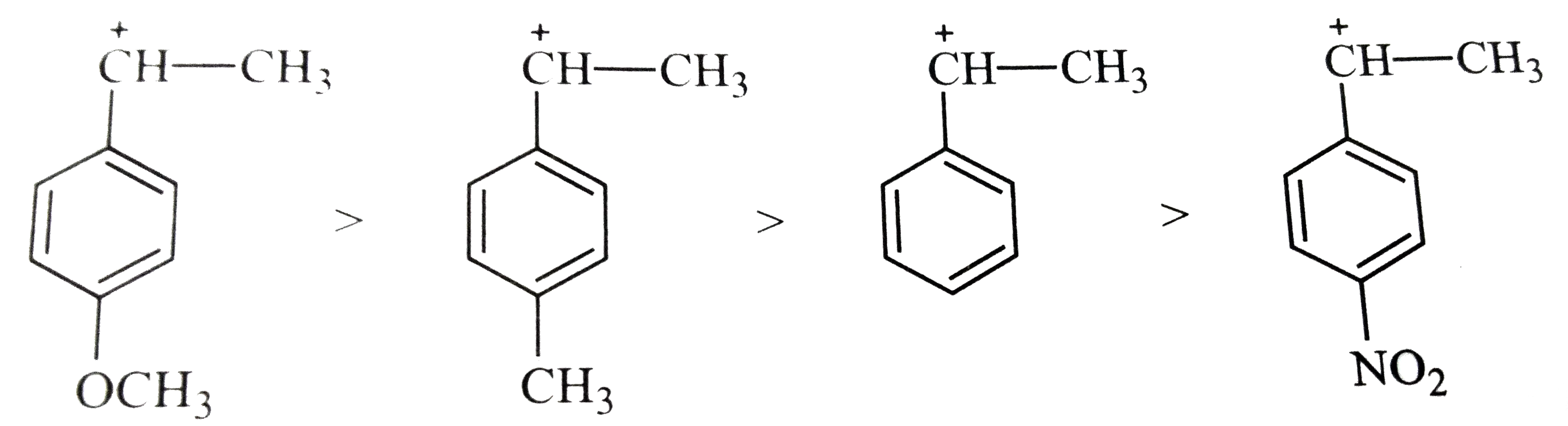
Text Solution
Verified by Experts
Topper's Solved these Questions
POLYMERS
PRADEEP|Exercise Value Based Questions with answers|5 VideosPOLYMERS
PRADEEP|Exercise Competition Focus (JEE (main and Advanced) /Medical Entrance Special) (I. Multiple Choice Questions) (with one Correct Answer)|44 VideosPOLYMERS
PRADEEP|Exercise Additional Questions (LONG ANSWER QUESTIONS)|3 VideosP-BLOCK ELEMENTS
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATION|25 VideosREDOX REACTIONS
PRADEEP|Exercise Assertion reason type question|16 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-POLYMERS-HIGHER ORDER THINKING SKILLS (Questions and problems with answers/solutions (Hots Questions)
- Arrange the following alkenes towards order of increasing reactivity i...
Text Solution
|
- Arrange the following monomers in order of decreasing reactivity in ca...
Text Solution
|
- Arrange the following alkenes in order of increasing reactivity toward...
Text Solution
|
- Will you prefer to polymerize acrylonitrile under anionic or cationic ...
Text Solution
|
- Free radical polymerization of stryene gives a product in which groups...
Text Solution
|
- What are chain transfer agents ? Giving a suitable example , explain t...
Text Solution
|
- Explain why vinylidene chloride (CH2=C CI2) does not polymerise is iso...
Text Solution
|
- Polypropylene contains a large number of chiral carbon atoms. Would yo...
Text Solution
|
- Explain how does 1, 3-butadiene polymerise by different route
Text Solution
|
- What is nylon ? Write an equation for the chemistry involved when a dr...
Text Solution
|
- A regular copolymer of ethylene and vinyl chloride contains alternate ...
Text Solution
|
- Give the structures of the products .
Text Solution
|
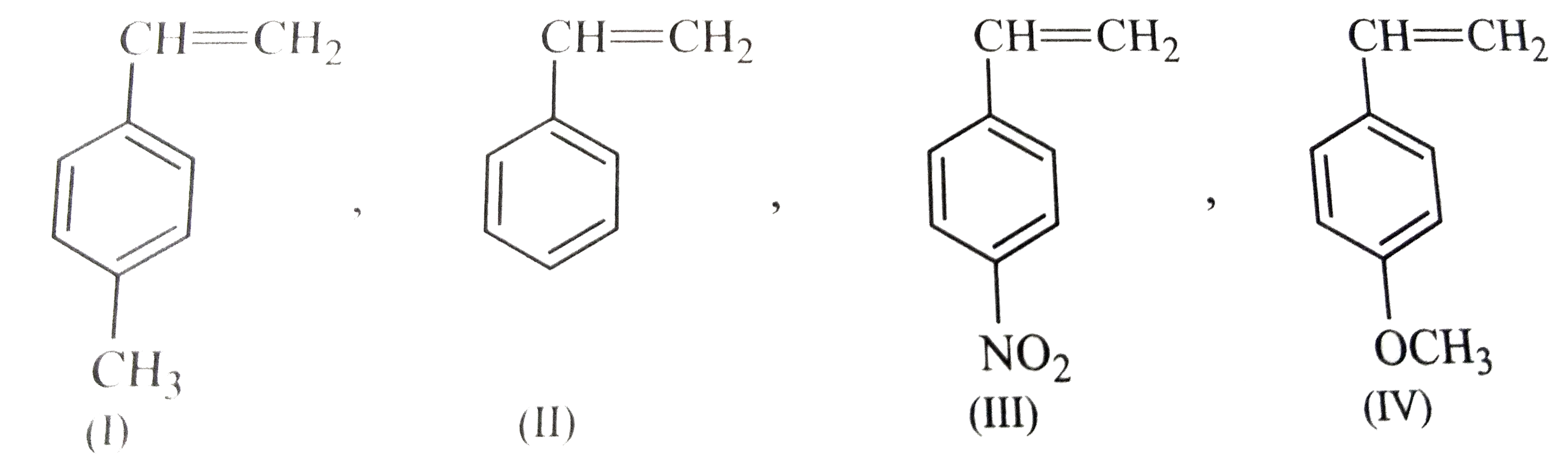 .
.