Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PRADEEP-SOME BASIC CONCEPTS OF CHEMISTRY-Competition (FOCUS) JEE (Main and Advanced)/Medical Entrance SPECIAL (VIII. Assertion-Reason Type Questions)(Type II)
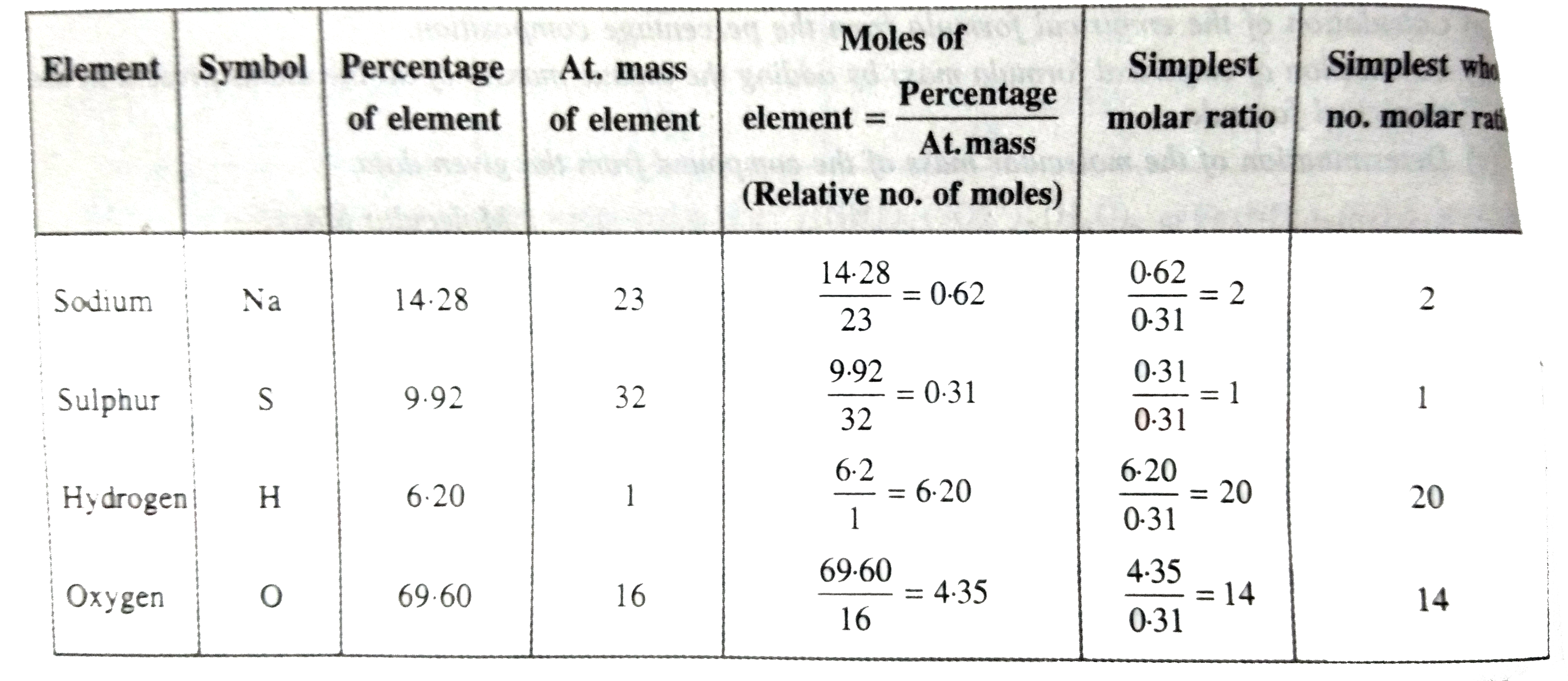
- A compound on analysis was found to contain the following composition ...
Text Solution
|
- Assertion. Phenol is a disinfectant. Reason. Disinfectants are used ...
Text Solution
|
- Assertion. Cinnabar is a chemical compound whereas brass is a mixture....
Text Solution
|
- Assertion (A): pure water obtained from different states of india alwa...
Text Solution
|
- Assertion. The size of a degree on Fahrenheit scale is smaller than th...
Text Solution
|
- Assertion. The number 14.56pm0.01 has three significant figures. Rea...
Text Solution
|
- Assertion. Gay Lussac's law does not follow from Dalton's atomic theor...
Text Solution
|
- Assertion. Average atomic mass of an element depends mainly on the hea...
Text Solution
|
- Statement-1 : Atomic mass of sodium is 23u Statement-2 : An atom of...
Text Solution
|
- Assertion. Both 106 g of sodium carbonate and 12 g of carbon have same...
Text Solution
|
- Assertion : Equivalent weight of a base =("Molecular weight")/("Acidit...
Text Solution
|
- Assertion: Equal moles of different substnaces contains same number of...
Text Solution
|
- Assertion: Empirical and molecular formula of Na2CO3 is same. Reason:...
Text Solution
|
- Assertion. In a combustion reaction in the air, oxygen is the limiting...
Text Solution
|