Text Solution
Verified by Experts
Topper's Solved these Questions
SOME BASIC CONCEPTS OF CHEMISTRY
PRADEEP|Exercise ADVANCED PROBLEMS (FOR COMPETITIONS)|17 VideosSOME BASIC CONCEPTS OF CHEMISTRY
PRADEEP|Exercise TEST YOUR GRIP (MULTIPLE CHOICE QUESTIONS)|20 VideosSOME BASIC CONCEPTS OF CHEMISTRY
PRADEEP|Exercise CURIOSITY QUESTION|6 VideosSOLUTIONS
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATION|31 VideosSURFACE CHEMISTRY
PRADEEP|Exercise IMPORTANT QUESTIONS (FOR BOARD EXAMINATION)|28 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-SOME BASIC CONCEPTS OF CHEMISTRY-PROBLEMS FOR PRACTICE
- A chemical compound is found to have the following composition : C=1...
Text Solution
|
- Butyric acid contains only C, H and O. A 4.24 mg sample of butyric aci...
Text Solution
|
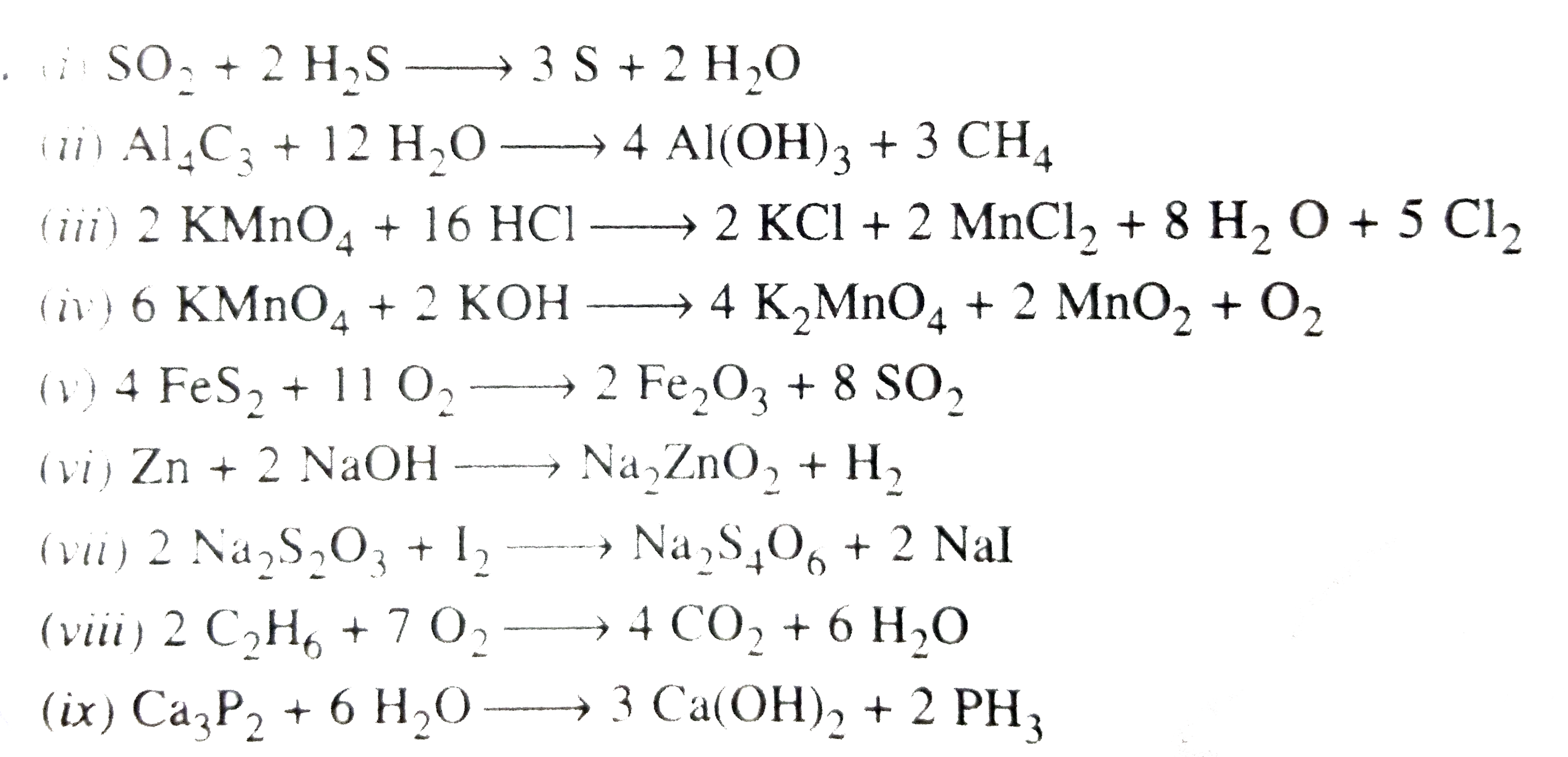
- Balance the following equations by Hit and Trial Method : (i) SO(2)+...
Text Solution
|
- Balance the following equation by partial Equation method: (i) PbS+O...
Text Solution
|
- In the commercial manufacture of nitric acid, how many moles of NO(2)...
Text Solution
|
- How much iron can be theoretically obatined by the reduction of 1.0kg ...
Text Solution
|
- Calculate the weight of 60% H(2)SO(4) required decomposing 50 g of cha...
Text Solution
|
- Which is cheaper : 40% hydrochloric acid at the rate of 50 paise per k...
Text Solution
|
- Excess of AgNO(3) solution was added to 2.2 g of commercial sample of ...
Text Solution
|
- A sample of dolomite contained 45% of CaCO(3), 40% of MgCO(3) and 15% ...
Text Solution
|
- Calculate the mass of graphite that must be burnt to produce 13.2 g o...
Text Solution
|
- One gram of a mixture of potassium and sodium chlorides on treatment w...
Text Solution
|
- What volume of oxygen at 18^(@)C and 750 mm pressure can be obtained f...
Text Solution
|
- What mass of iodine is liberated from a solution of potassium iodide w...
Text Solution
|
- 1.4 g of a sample of chalk (CaCO(3)) containing clay as impurity were...
Text Solution
|
- How much marble of 96.5% purity would be required to prepare 10 litres...
Text Solution
|
- Calculate the volume of SO(2) at STP obtained by burning 500 g of S co...
Text Solution
|
- 2.5 g of an impure sample of sodium bicarbonate when heated strongly g...
Text Solution
|
- 10 mL of liquid carbon disulphide (specific gravity 2.63) is burnt is ...
Text Solution
|
- The drain cleaner, Drainex contains small bits of aluminium which reac...
Text Solution
|