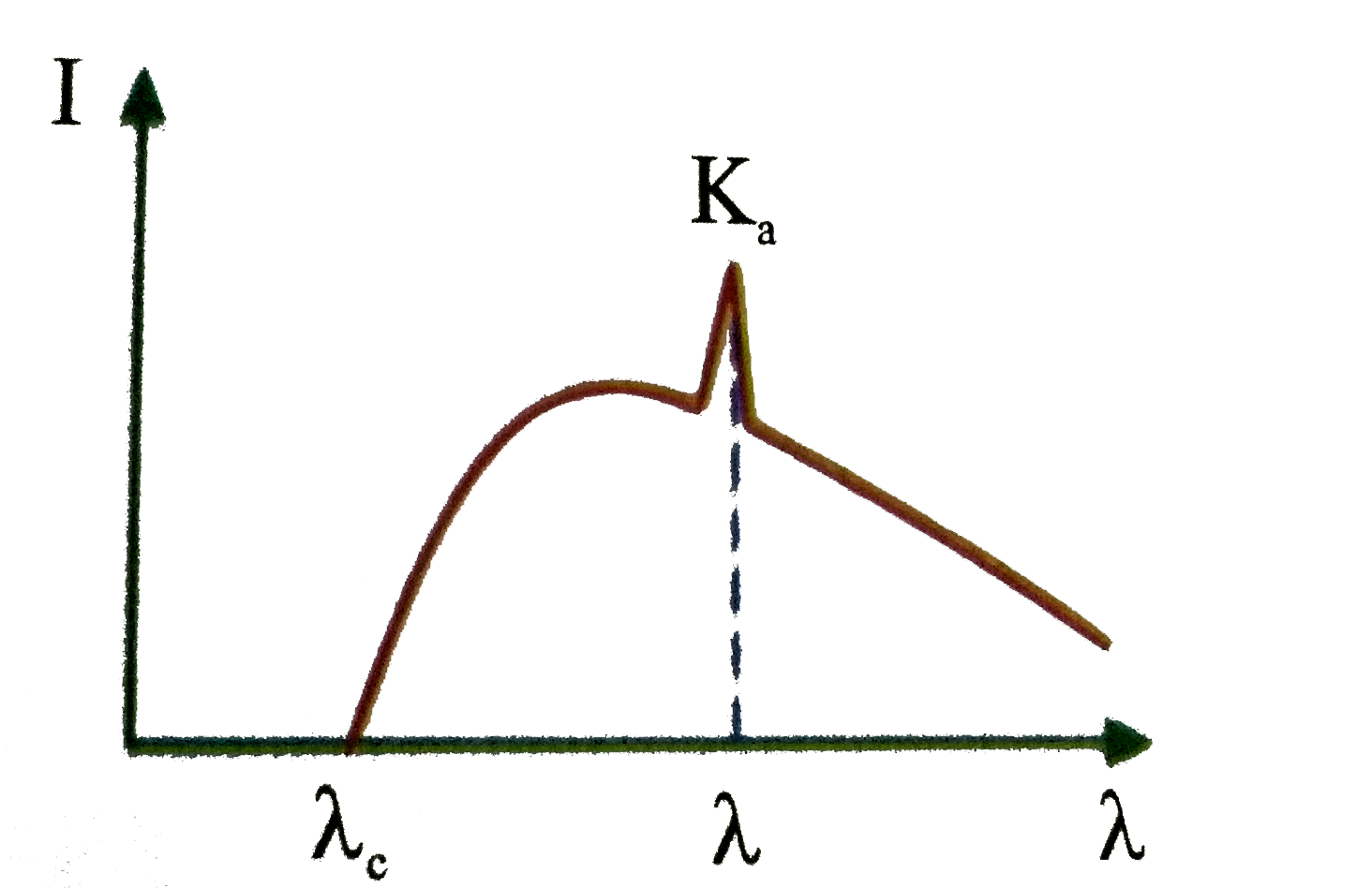
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-ATOMIC PHYSICS-LEVEL-III
- Assertion : An electron in hydrogen in hydrogen atom passes from n=4 t...
Text Solution
|
- An H atom in ground state is moving with initial kinetic energy K .It ...
Text Solution
|
- Given X-ray spectrum is for a coolidge tube having accelerating potent...
Text Solution
|
- The innermost orbit of the hydrogen atom has a diameter of 1.06Å .What...
Text Solution
|
- The energy different between the first two levels of hydrogen atom is ...
Text Solution
|
- An energy of 24.6 eV is required to remove one of that electrons from ...
Text Solution
|
- Which energy state of doubly ionized lithium Li^(++) has the same ener...
Text Solution
|
- If an orbital electron of the hydrogen atom jumps from the groud state...
Text Solution
|
- The relation between lambda(1)= wavelength of series limit of Lyman se...
Text Solution
|
- According to Bohr's theory of the hydrogen atom , the speed v(n) of t...
Text Solution
|
- The orbital speed of the electron in the ground state of hydrogen is v...
Text Solution
|
- In the Bohr model of the hydrogen atom, the ratio of the kinetic energ...
Text Solution
|
- The total energy of an electron in the first excited state of hydrogen...
Text Solution
|
- The total energy of an electron in the first excited state of hydrogen...
Text Solution
|
- The highest energy state , that unexcited hydrogen atoms can reach whe...
Text Solution
|
- In the spectrum of hydrogen atom, the ratio of the longest wavelength ...
Text Solution
|
- The frequency of the first line in Lyman series in the hydrogen spect...
Text Solution
|
- The electron in a hydrogen atom makes a transition from an excited sta...
Text Solution
|
- Three photons coming from excited atoms hydrogen sample are picked up ...
Text Solution
|
- Ionization potential of hydrogen atom is 13.6 V. Hydrogen atoms in the...
Text Solution
|