A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
NARAYNA-ATOMIC PHYSICS-LEVEL-VI
- The properties of x -rays as put forwarded by Rontgen in his pioneerin...
Text Solution
|
- The properties of x -rays as put forwarded by Rontgen in his pioneerin...
Text Solution
|
- The properties of x -rays as put forwarded by Rontgen in his pioneerin...
Text Solution
|
- A positronium atom consist of an electron and a positron revolving abo...
Text Solution
|
- A positronium atom consist of an electron and a positron revolving abo...
Text Solution
|
- A neutrons beam, in which each neutron has same Kinetic energy , is pa...
Text Solution
|
- A neutrons beam, in which each neutron has same Kinetic energy , is pa...
Text Solution
|
- If an electrons jumps from m^(th) orbit to the nth orbit (mgtn) the en...
Text Solution
|
- If an electrons jumps from m^(th) orbit to the nth orbit (mgtn) the en...
Text Solution
|
- A beam of alpha paricles is incident on a target of lead. A particular...
Text Solution
|
- A beam of alpha paricles is incident on a target of lead. A particular...
Text Solution
|
- A beam of alpha paricles is incident on a target of lead. A particular...
Text Solution
|
- In each of the following questions, a statement is given and a corresp...
Text Solution
|
- Statement-2: When a beam of highly energetic neutrons is incident on a...
Text Solution
|
- Assertion: In the duration electron jumps from fist excited state to g...
Text Solution
|
- Statement -1: In process of photoelectric emission , all emitted elect...
Text Solution
|
- Statement-1: The de-Broglie wavelength of a molecules (in a sample of ...
Text Solution
|
- Statement -1: When applied potential difference Between cathode and an...
Text Solution
|
- Statement-1: In Photoelectric effect , electrons absorbing the photon ...
Text Solution
|
- In Fig. electromagnetic radiations of wavelength 200nm are incident on...
Text Solution
|
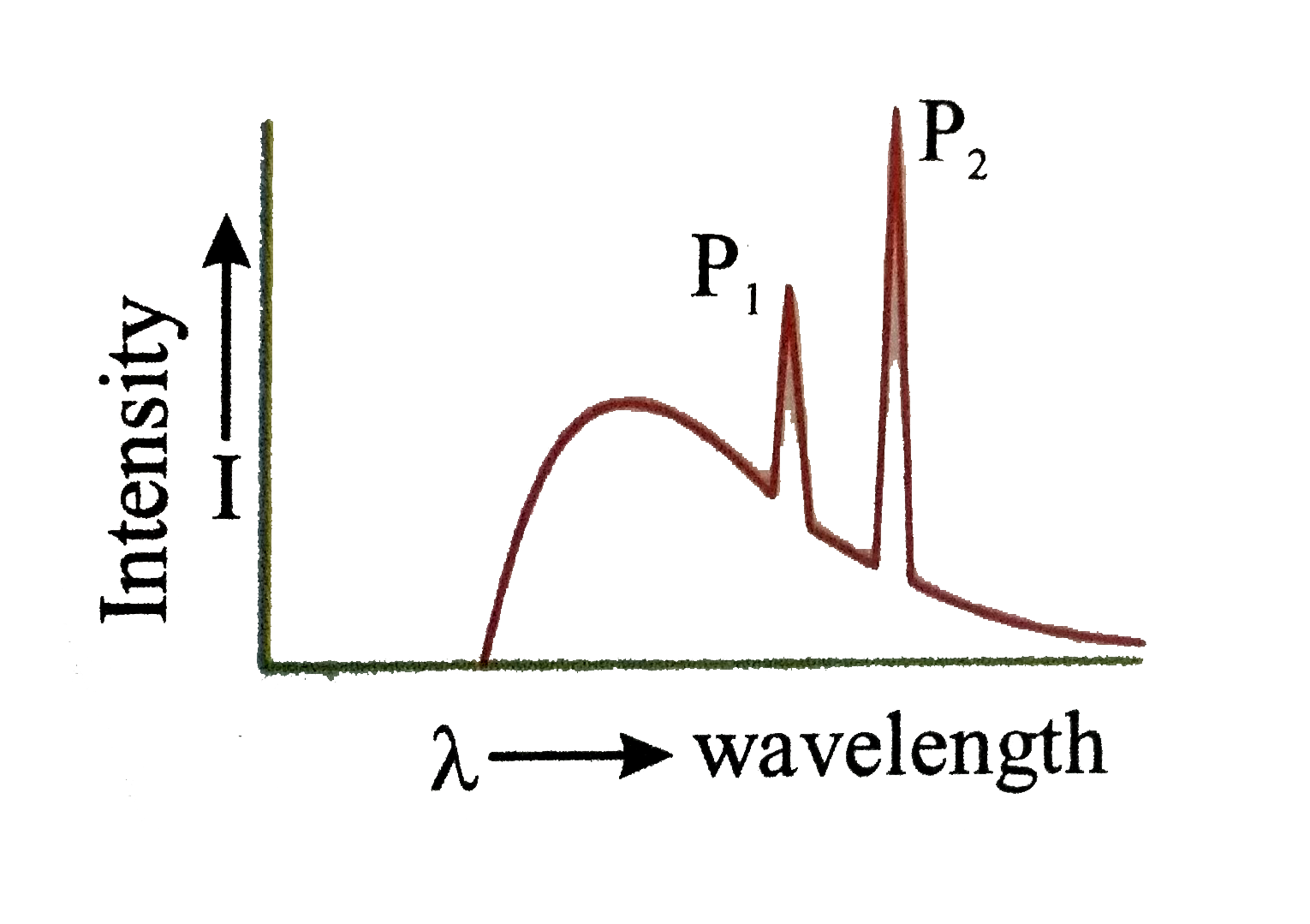 The electron knocks out an inner shell electron of the atom with which it collides . Let us take an hypothetical case of a target atom whose K- shell electron has been knocked out as shown.
The electron knocks out an inner shell electron of the atom with which it collides . Let us take an hypothetical case of a target atom whose K- shell electron has been knocked out as shown. 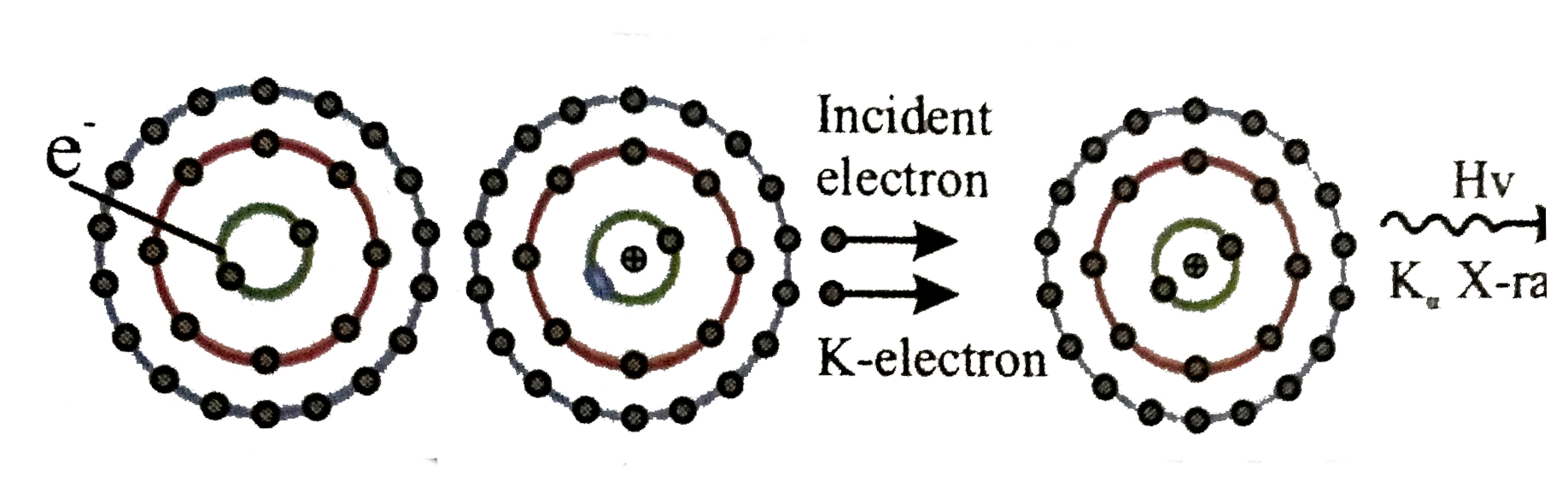 this will create a vacancy in K-shell . sensing this vacancy an electron from a higher energy state may make a trasition to thsi vacant state. when such a transition take placce the differece of energy is converted into photon of electromagnetic radiation, which is called charactertistic `X`-rays . Now depending upon the shell from which an electron make a trasition to `K` -shell we may have different lines in the `K` series of `X`rays e.g. if electron from L shell jumps to `K` shell we have K_(alpha), if electron from M shell jumps to `K` shell we have `K_(beta)` `X` -rays and so . on . Monseley conducted many experiment on characteristic `X`-rays , the finding of whcih played an inportant role in . developing the concept opf atomic number . Moseley's observation can be expressed as `sqrtv=a(Z-b)`
this will create a vacancy in K-shell . sensing this vacancy an electron from a higher energy state may make a trasition to thsi vacant state. when such a transition take placce the differece of energy is converted into photon of electromagnetic radiation, which is called charactertistic `X`-rays . Now depending upon the shell from which an electron make a trasition to `K` -shell we may have different lines in the `K` series of `X`rays e.g. if electron from L shell jumps to `K` shell we have K_(alpha), if electron from M shell jumps to `K` shell we have `K_(beta)` `X` -rays and so . on . Monseley conducted many experiment on characteristic `X`-rays , the finding of whcih played an inportant role in . developing the concept opf atomic number . Moseley's observation can be expressed as `sqrtv=a(Z-b)`