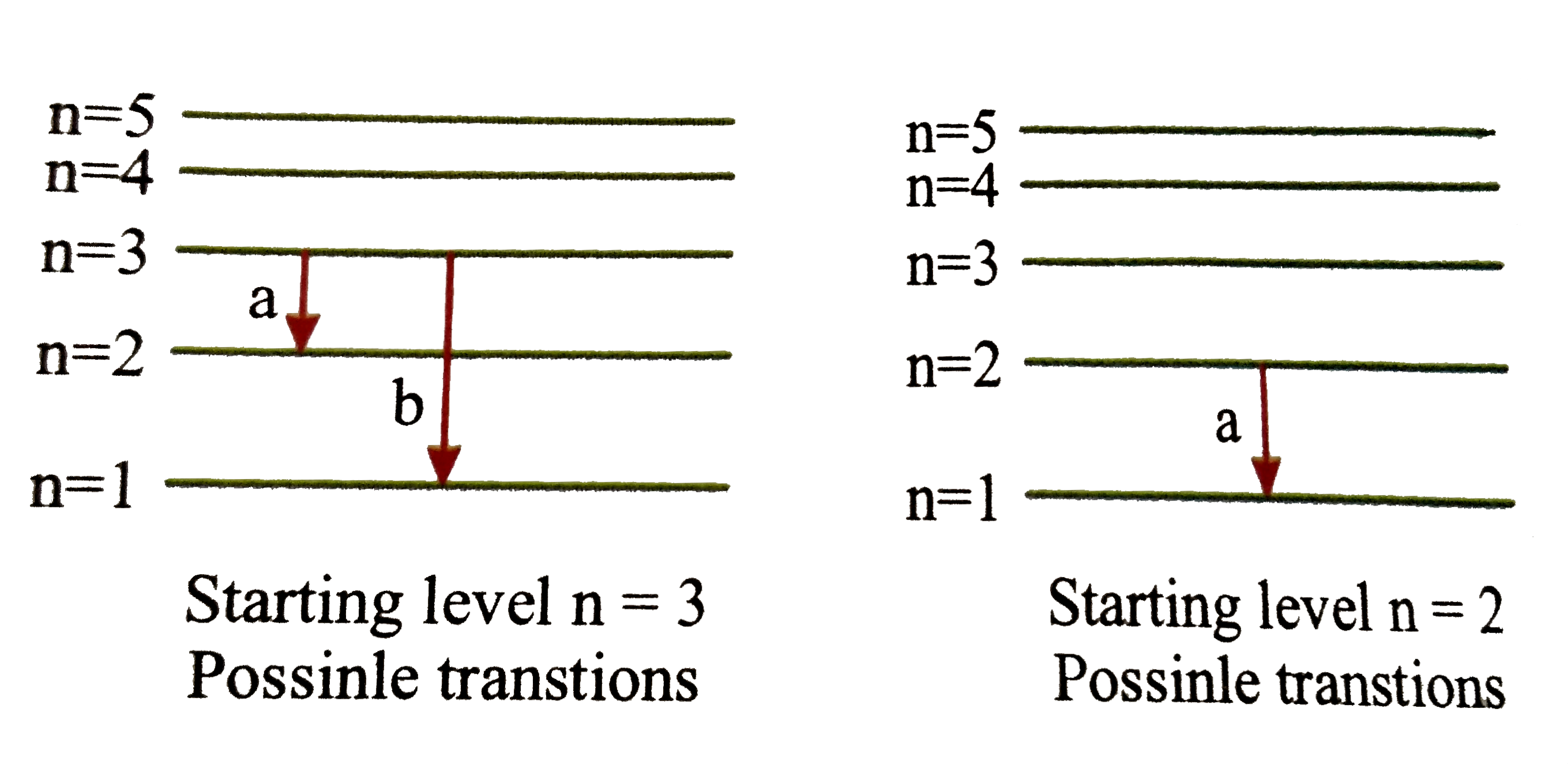Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-ATOMIC PHYSICS-LEVEL-VI
- If an electrons jumps from m^(th) orbit to the nth orbit (mgtn) the en...
Text Solution
|
- If an electrons jumps from m^(th) orbit to the nth orbit (mgtn) the en...
Text Solution
|
- A beam of alpha paricles is incident on a target of lead. A particular...
Text Solution
|
- A beam of alpha paricles is incident on a target of lead. A particular...
Text Solution
|
- A beam of alpha paricles is incident on a target of lead. A particular...
Text Solution
|
- In each of the following questions, a statement is given and a corresp...
Text Solution
|
- Statement-2: When a beam of highly energetic neutrons is incident on a...
Text Solution
|
- Assertion: In the duration electron jumps from fist excited state to g...
Text Solution
|
- Statement -1: In process of photoelectric emission , all emitted elect...
Text Solution
|
- Statement-1: The de-Broglie wavelength of a molecules (in a sample of ...
Text Solution
|
- Statement -1: When applied potential difference Between cathode and an...
Text Solution
|
- Statement-1: In Photoelectric effect , electrons absorbing the photon ...
Text Solution
|
- In Fig. electromagnetic radiations of wavelength 200nm are incident on...
Text Solution
|
- Consider Bohr's theory for hydrogen atom. The magnitude of angular mom...
Text Solution
|
- An gas of H-atom in excited state n(2) absorbs a photon of some energy...
Text Solution
|
- Hydrogen aton in a sample are excited to n = 5 state and it is found t...
Text Solution
|
- A proton is fired from very far away towards a nucleus with charge Q ...
Text Solution
|
- The average life time of an excited state of an electron in Hydrogen i...
Text Solution
|
- A monochromatic light soure of frequency f illuminates a metallic sur...
Text Solution
|
- A small particle of mass m move in such a way the potential energy (U ...
Text Solution
|