A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-ATOMIC PHYSICS-LEVEL-I (H.W)
- Two electrons Beams having velocities in the ratio1:2 are subjected to...
Text Solution
|
- The Velocity of electrons accelerated bt potential difference of 1xx10...
Text Solution
|
- Cathode ray tube is operating at5KV. . Then the K.E. acquire by the el...
Text Solution
|
- A steam of similar negatively charged particles enters an electrical f...
Text Solution
|
- An oil drop having a mass 4.8xx10^(-10)g. and charge 2.4xx10^(-18)C st...
Text Solution
|
- In Millikan oil drop experiment, a charged drop of mass 1.8 xx 10^(-14...
Text Solution
|
- An alpha-particle accelerated through V volt is fired towards a nucleu...
Text Solution
|
- In Rutherford scattering experiment, what will b ethe correct angle fo...
Text Solution
|
- The impact parameter at which the scattering angle is 90^(0) , z=79 an...
Text Solution
|
- If a hydrogen atom emit a photon of energy 12.1 eV , its orbital angul...
Text Solution
|
- In an excited state of hydrogen like atom an electron has total energy...
Text Solution
|
- What is the ionisation potential of hydrogen atom?
Text Solution
|
- The energy of electron in an excited hydrogen atom is -3.4eV. Its angu...
Text Solution
|
- The velocity of an electron in the second orbit of sodium atom (atomic...
Text Solution
|
- In any Bohr orbit of the hydrogen atom, the ratio of kinetic energy to...
Text Solution
|
- Let the potential energy of the hydrogen atom in the ground state be z...
Text Solution
|
- The value of wavelength radiation emitted by an H-atom, if atom is exc...
Text Solution
|
- the maximum number of photons emitted by an H-atom , if atom is excite...
Text Solution
|
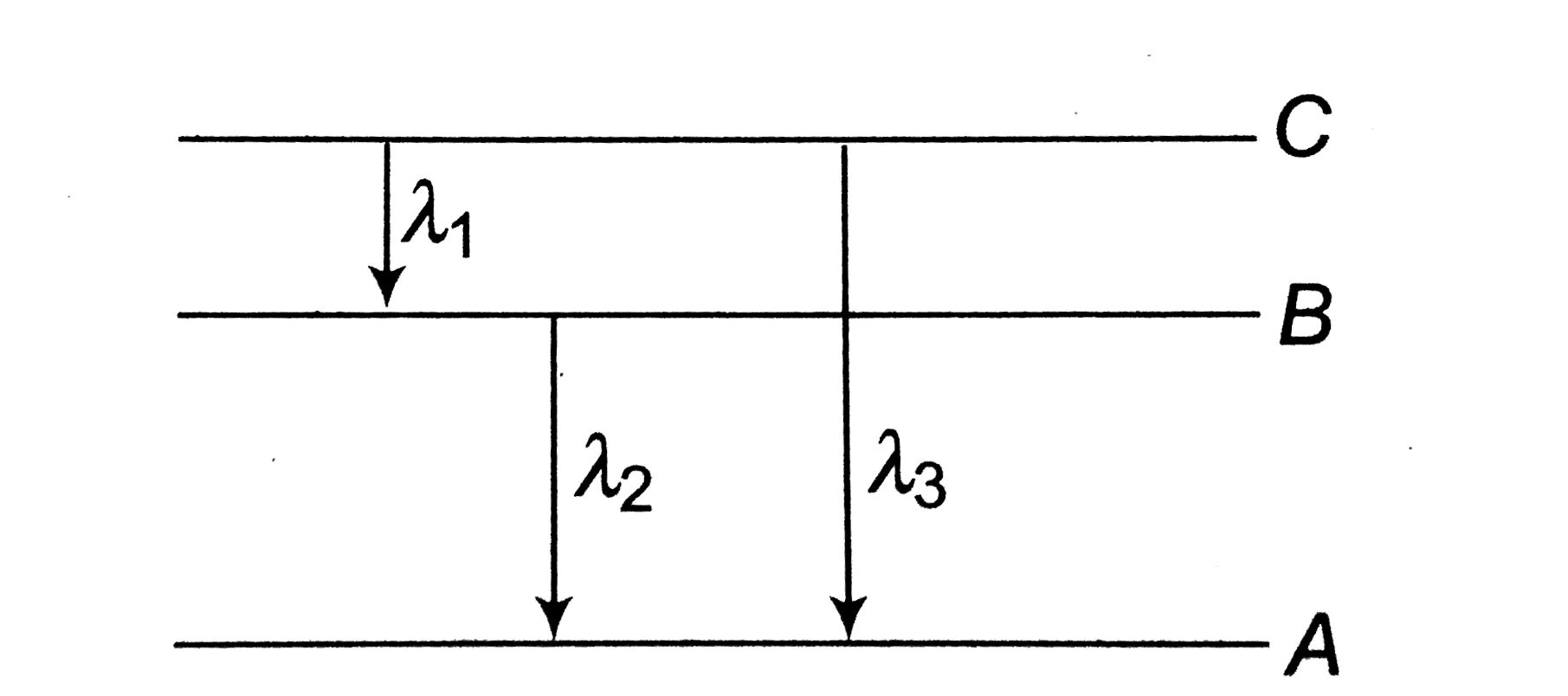
- Energy levels A, B, C of a certain atom corresponding to increasing va...
Text Solution
|
- It is found experimentally that 13.6 eV energy is required to separate...
Text Solution
|