Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-KINETIC THEORY OF GASES-SOLVED EXAMPLE
- A quantity of heat Q is supplied to a monoatomic ideal gas which expan...
Text Solution
|
- The specific heat capacity of a metal at low temperature (T) is given ...
Text Solution
|
- The above p-v diagram represents the thermodynamic cycle of an engine,...
Text Solution
|
- A piston divides a closed gas cylinder into two parts. Initially the p...
Text Solution
|
- In a cyclic process shown in the figure an ideal gas is adiabatically...
Text Solution
|
- A gas undergoes a change of state during which 100J of heat is supplie...
Text Solution
|
- An ideal gas is taken through the cycle AtoBtoCtoA, as shown in the fi...
Text Solution
|
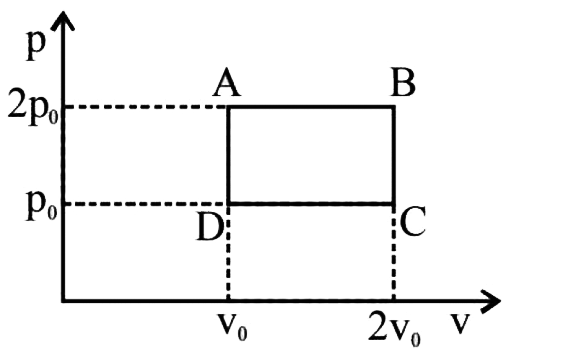
- An ideal monoatomic gas is taken round the cycle ABCDA as shown in the...
Text Solution
|
- During an adiabatic process, the pressure of a gas is found to be prop...
Text Solution
|
- When 5 moles of an ideal gas is compressecd isothermally, its volume d...
Text Solution
|
- A gas is expanded to double its volume by two different processes. One...
Text Solution
|
- Temperature of 1 mole of an ideal gas is increased from 300K to 310K u...
Text Solution
|
- A tyre pumped to a pressure of 6 atmosphere suddenly bursts. Room temp...
Text Solution
|
- Three samples of the same gas A,B and C (gamma=3//2) have initially eq...
Text Solution
|
- An ideal gas mixture filled inside a balloon expands according to the ...
Text Solution
|
- Find the amount of work done to increase the temperature of one mole j...
Text Solution
|
- P-V diagram of a diatomic gas is a straight line passing through origi...
Text Solution
|
- The molar heat capacity in a process of a diatomic gas if it does a wo...
Text Solution
|
- A monoatomic gas undergoes a process given by 2dU+3dW=0, then what is ...
Text Solution
|
- The relation between U, P and V for an iodeal gas is U=2+3PV. What is ...
Text Solution
|