Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-KINETIC THEORY OF GASES-SOLVED EXAMPLE
- 3 moles of an ideal mono atomic gas performs a cycle as shown in fig. ...
Text Solution
|
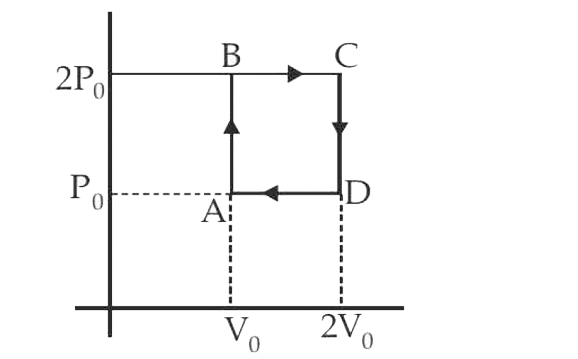
- An ideal gas is subjected to a cyclic process ABCD as depicted in the ...
Text Solution
|
- A refrigerator, whose coefficient performance beta is 5, extracts heat...
Text Solution
|
- A carnot engine operating between temperatures T(1) and T(2) has effic...
Text Solution
|
- Find the efficeincy of the thermodynamic cycle shown in figure for an ...
Text Solution
|
- A Carnot's engine whose sink is at temperature of 300K has an efficien...
Text Solution
|
- A Carnot engine, having an efficiency of eta=1//10 as heat engine, is ...
Text Solution
|
- Helium gas goes through a cycle ABCDA (consisting of two isochoric and...
Text Solution
|
- A Carnot engine, whose efficiency is 40%, takes in heat from a source ...
Text Solution
|
- A diatomic ideal gas is used in a Carnot engine as the working substan...
Text Solution
|
- An ideal monoatomic gas undergoes a cyclic process ABCA as shown in th...
Text Solution
|
- 3 moles of an ideal mono atomic gas performs a cycle as shown in fig. ...
Text Solution
|
- P - V diagram of an ideal gas is as shown in figure. Work done by the ...
Text Solution
|
- Volume versus temperature graph of two moles of helium gas is as shown...
Text Solution
|
- Pressure versus temperature graph of an ideal gas is shown in figure. ...
Text Solution
|
- In the P-V diagram shown in figure ABC is a semicircle. The work done ...
Text Solution
|
- Pressure versus temperature graph of an ideal gas as shown in Fig. C...
Text Solution
|
- A thermodynamic system undergoes cyclic process ABCDA as shown in figu...
Text Solution
|
- P-V plots for two gases during adiabatic processes are shown in the fi...
Text Solution
|
- One mole of a diatomic ideal gas undergoes a cyclic process ABC as sho...
Text Solution
|