A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-KINETIC THEORY OF GASES-LEVEL-III(C.W)
- A Carnot's engine is made to work between 200^(@)C and 0^(@)C first an...
Text Solution
|
- A scientist says that the efficiency of his heat engine which operates...
Text Solution
|
- Efficiency of a Carnot engine is 50% when temperature of outlet is 500...
Text Solution
|
- An ideal refrigerator has a freezer at a temperature of -13^(@)C. The ...
Text Solution
|
- The heat reservoir of an ideal carnot engine is at 800K and its sink i...
Text Solution
|
- A Carnot engine workds between 200^(@)C and 0^(@)C and .-200^(@)C. In ...
Text Solution
|
- In the above problem, the output of second engine is
Text Solution
|
- In the above problem, the ratio of outputs of two engines is
Text Solution
|
- A carbot freezer takes heat from water at 0^(@)C inside it and rejects...
Text Solution
|
- A carnot engine absorbs 1000J of heat energy from a reservoir at 127^(...
Text Solution
|
- An ideal monoatomic gas is taken round the cycle ABCDA as shown in the...
Text Solution
|
- The figure shows P-V graph of an ideal on molegas undergone to cyclic ...
Text Solution
|
- On a T-P diagram, two moles of ideal gas perform process AB and CD. If...
Text Solution
|
- A sample of an ideal monoatomic gas is taken round the cycle ABCA as s...
Text Solution
|
- In the given elliptical P-V diagram
Text Solution
|
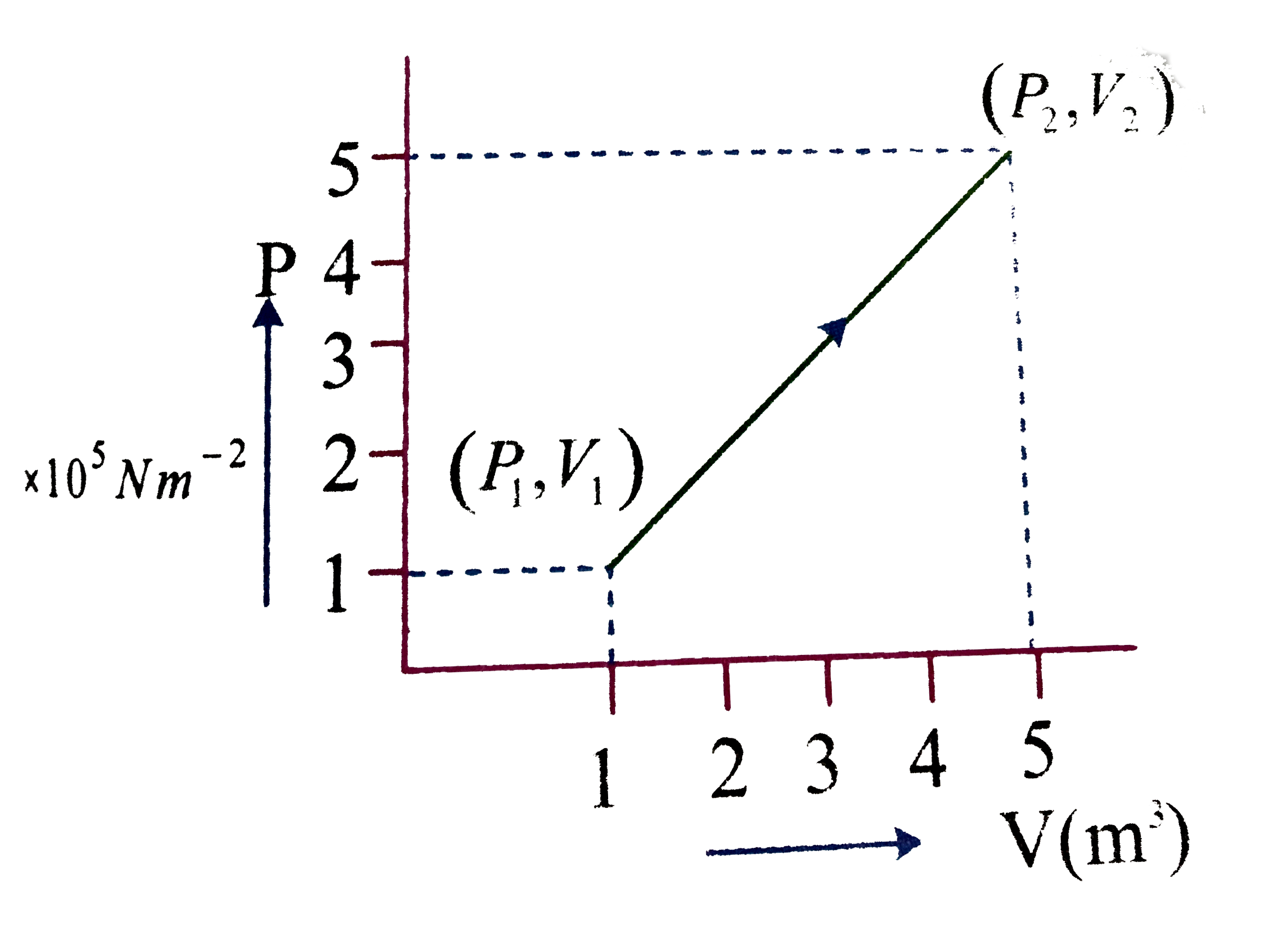
- A system changes from the state (P(1),V(1)) to (P(2)V(2)) as shwon in ...
Text Solution
|
- Heat energy abosrbed by a system in going through a cyclic process sho...
Text Solution
|
- A thermodynamic system is taken through the cyclic PQRSP process. The ...
Text Solution
|
- A cyclic process performed on one mole of an ideal gas. A total 1000 J...
Text Solution
|
- An ideal gas is taken through the cycle AtoBtoCtoA, as shown in the fi...
Text Solution
|