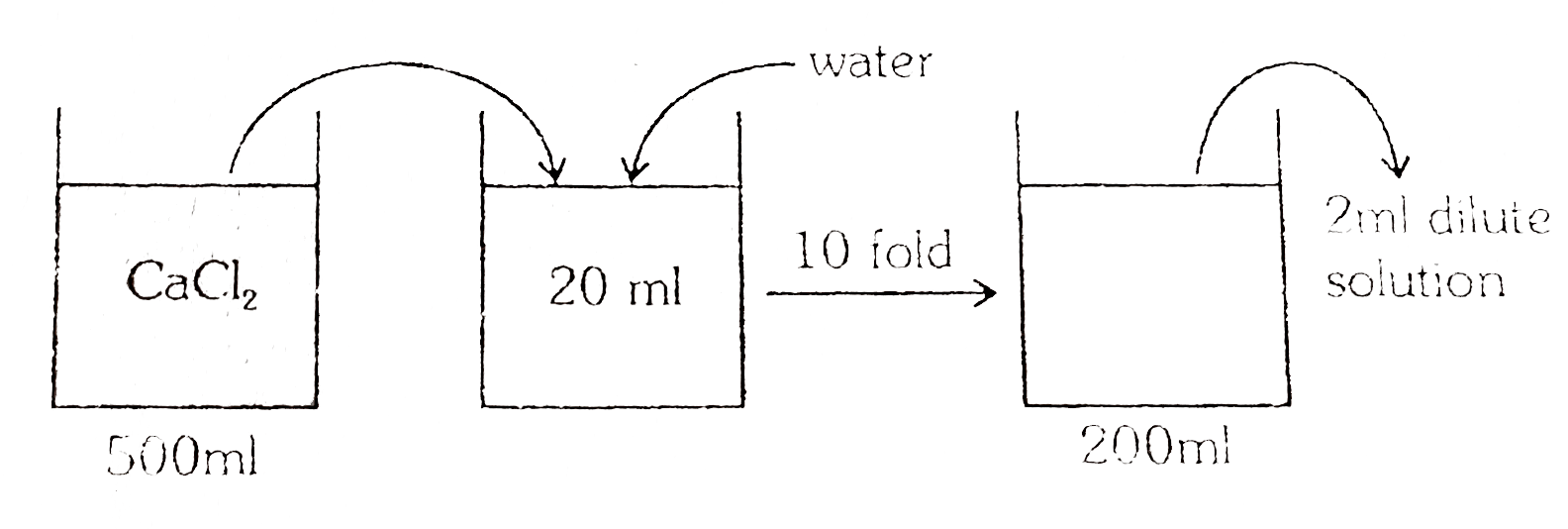Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-MOLE CONCEPT-Exercise - 05(B)
- 1.11g CaCl(2) is added to water forming 500ml of solution 20ml of this...
Text Solution
|
- At 100^(@)C and 1 atm, if the density of the liquid water is 1.0 g cm^...
Text Solution
|
- How many moles of electrons weigh 1 kg?
Text Solution
|
- Calculate the molarity of water if its density is 1000 kg m^(-3)
Text Solution
|
- 1 g charcoal is placed in 100 mL of 0.5 M CH(3)COOH to form an adsorbe...
Text Solution
|
- Calculate the amount of calcium oxide required when it reacts with 852...
Text Solution
|
- Around 20% surface sites have adsorbed N(2). On heating N(2) gas evolv...
Text Solution
|
- Given that the abundacne of isotopes .^(54)Fe, .^(56)Fe, and .^(57)Fe ...
Text Solution
|
- Dissolving 120g of urea (Mw = 60) in 1000 g of water gave a solution o...
Text Solution
|