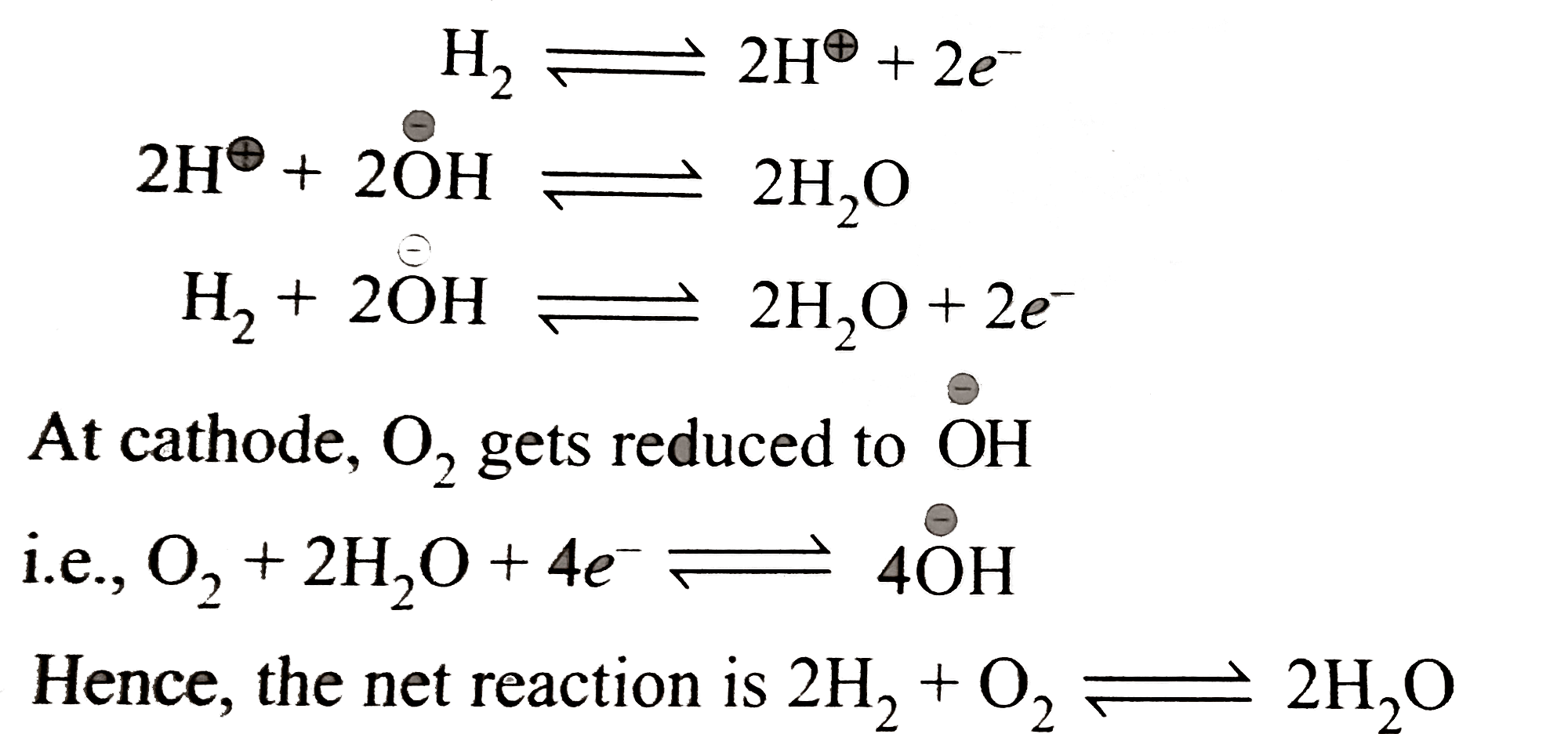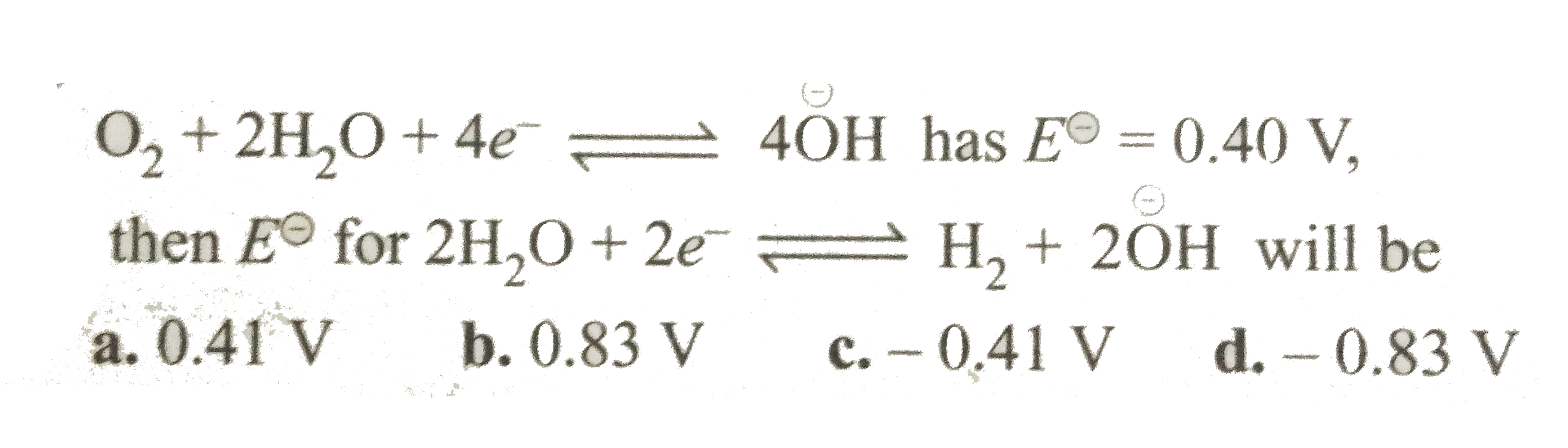A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-ELECTROCHEMISTRY-COMPREHENSION TYPE
- Fuel cells : Fuel cells are galvanic cells in which the chemical energ...
Text Solution
|
- Fuel cells : Fuel cells are galvanic cells in which the chemical energ...
Text Solution
|
- Fuel cells : Fuel cells are galvanic cells in which the chemical energ...
Text Solution
|
- One litre each of a Buffer solution is taken in two beakers connected ...
Text Solution
|
- One litre each of a Buffer solution is taken in two beakers connected ...
Text Solution
|
- One litre each of a Buffer solution is taken in two beakers connected ...
Text Solution
|
- One ecologically important equilibrium is that between carbonate and h...
Text Solution
|
- One ecologically important equilibrium is that between carbonate and h...
Text Solution
|
- One ecologically important equilibrium is that between carbonate and h...
Text Solution
|
- The magnitude ( but not the sign ) of the standard reduction potential...
Text Solution
|
- The magnitude ( but not the sign ) of the standard reduction potential...
Text Solution
|
- The magnitude ( but not the sign ) of the standard reduction potential...
Text Solution
|
- Given: E(Zn^(+2)//Zn)^(@) =- 0.76V E(Cu^(+2)//Cu)^(@) = +0.34V K...
Text Solution
|
- Given: E(Zn^(+2)//Zn)^(@) =- 0.76V E(Cu^(+2)//Cu)^(@) = +0.34V K...
Text Solution
|
- Given: E(Zn^(+2)//Zn)^(@) =- 0.76V E(Cu^(+2)//Cu)^(@) = +0.34V K...
Text Solution
|
- H(2)O(2) can be produced by the Ammonium hydrogen sulphate. Reactions ...
Text Solution
|
- H(2)O(2) can be produced by the ammonium hydrogen sulphate. Reactions ...
Text Solution
|
- H(2)O(2) can be produced by the Ammonium hydrogen sulphate. Reactions ...
Text Solution
|