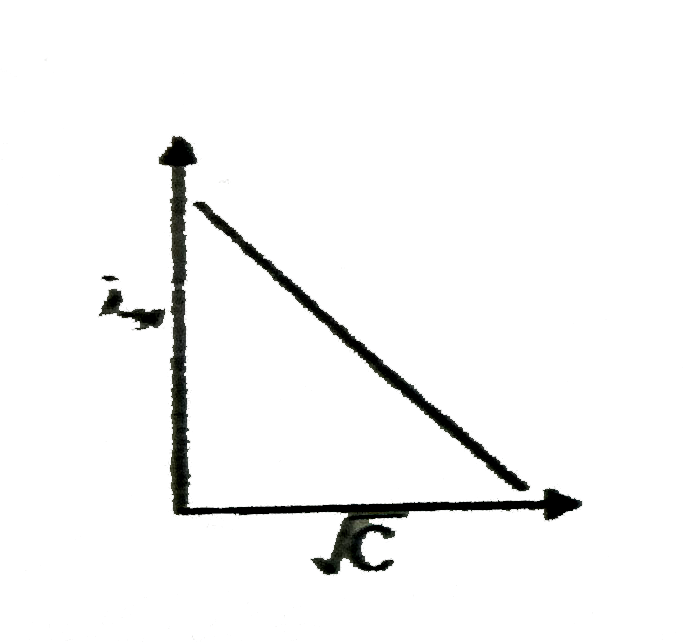
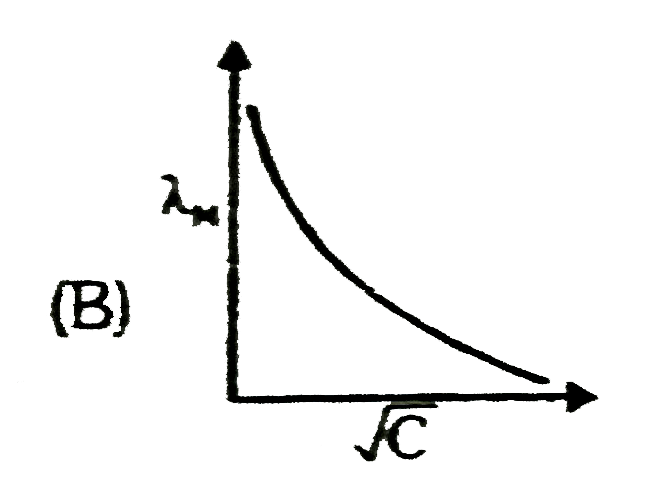
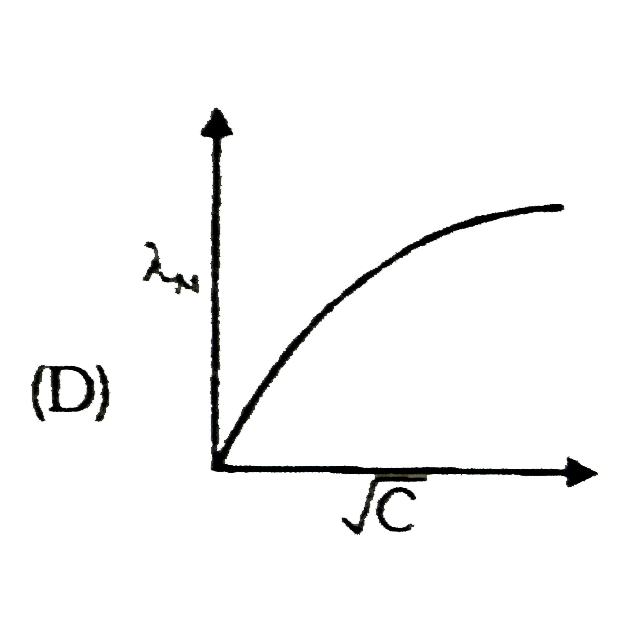
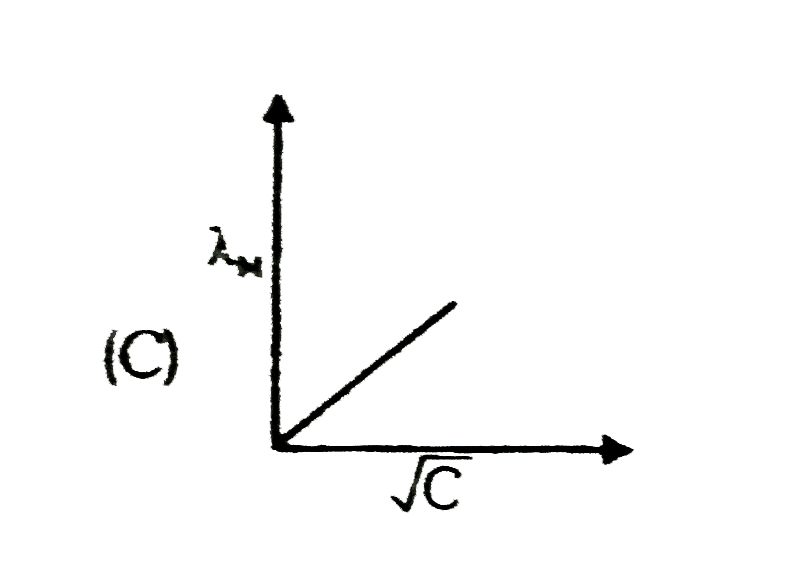A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-ELECTROCHEMISTRY-EXERCISE-02
- The reduction potential of a half-cell consisting of a Pt electrode im...
Text Solution
|
- Consider the cell |{:(H(2)(Pt)),(1atm):} :|: {:(H(3)O^(+)(aq)),(pH =5....
Text Solution
|
- Hg(2)CI(2) is produced by the electrolytic reduction of Hg^(2+) ion in...
Text Solution
|
- The ionization constant of a weak electrolyte is 2.5 xx 10^(-5), while...
Text Solution
|
- Which of the following curve represents the variation of lambda(M) wit...
Text Solution
|
- Four moles of electrons were transferred from anode to cathode in an e...
Text Solution
|
- Equivalent conductance of BaCI(2),H(2)SO(4) & HCI at infinite are A(oo...
Text Solution
|
- Salts of A (atomic weight 7), B (atomic weight 27) and C (atomic weigh...
Text Solution
|
- Durinh electrlysis of an aqueous solution of CuSO(4) using copper elec...
Text Solution
|
- The cost at 5 paise/KWH of operating an electric motor for 8 hours whi...
Text Solution
|
- When an aqueous solution of LiCl is electrolyzed using graphite electr...
Text Solution
|
- A silver wire dipped in 0.1M HCI solution saturated with AgCI develops...
Text Solution
|
- Consider the reaction fo extraction of gold from its ore Au +2CN^(-)...
Text Solution
|
- Consider the following Galvanic cell:- By what value the cell vol...
Text Solution
|
- For the fuel cell reaction 2H(2)(g) +O(2)(g) rarr 2H(2)O(l), Deta(f)H(...
Text Solution
|
- The resistance of 0.5M solution of an electrolyte in a cell was found ...
Text Solution
|
- The dissociation constant of n-butyric acid is 1.6 xx 10^(-5) and the ...
Text Solution
|
- A graph was plotted between the molar conductance of various electroly...
Text Solution
|
- Starting with 250 ml of Au^(3+) solution 250 ml of Fe^(2+) solution, t...
Text Solution
|
- The following data rae obtained in an experiment {:("Solution", "Con...
Text Solution
|