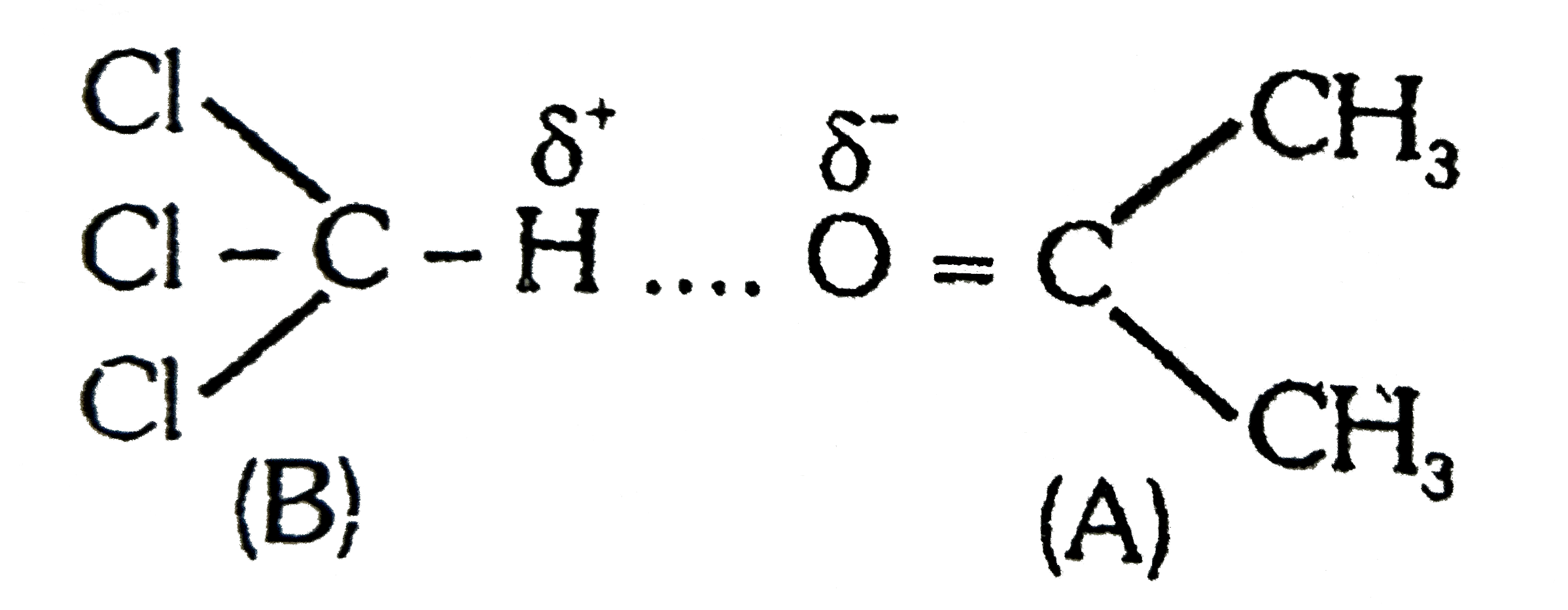Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-SOLUTIONS-EXERCISE-03
- {:(Column-I,Column-II),((a)"Acetone" +CHCI(3),(p)DeltaS gt 0),((b)"Eth...
Text Solution
|
- Assuming all the solutes are non volatile and all solutions are ideal.
Text Solution
|
- For a binary liquid solution of A and B. P^(overset(0)A)= pure vapour ...
Text Solution
|
- {:(Column-I,Column-II),("Colligative properties","Aqueous solution Ass...
Text Solution
|
- Statement-I: 0.1M solution of NaCI has greater osmotic pressure than 0...
Text Solution
|
- Statement-I: Relative lowering of vapour pressure is equal to mole fra...
Text Solution
|
- Statement-I : Molal elevation constant depends on the nature of solven...
Text Solution
|
- Statement-I: 0.02m solutions of urea and sucrose will freeze at same t...
Text Solution
|
- Statement-I: When mercuric iodide(s) is added to the aqueous solution ...
Text Solution
|
- Statement-I: 1M solution of Glauber's salt is isotonic with 1M solutio...
Text Solution
|
- Statement-I: If decimolal solution of sodium chloride boils at 101.2^(...
Text Solution
|
- Statement-1 : The freezing of water is an endothermic process. State...
Text Solution
|
- These questions consists of two statements each, printed as Statement-...
Text Solution
|
- Azoetropes are constant boiling mixtures, which like a pure chemical c...
Text Solution
|
- Azoetropes are constant boiling mixtures, which like a pure chemical c...
Text Solution
|
- Azoetropes are constant boiling mixtures, which like a pure chemical c...
Text Solution
|
- 10 mole of liquid 'A' and 20 mole of liquid 'B' is mixed in a cylindri...
Text Solution
|
- 10 mole of liquid 'A' and 20 mole of liquid 'B' is mixed in a cylindri...
Text Solution
|
- 10 mole of liquid 'A' and 20 mole of liquid 'B' is mixed in a cylindri...
Text Solution
|
- Acetic acid tends to form dimer due to formation of intermolcular hydr...
Text Solution
|