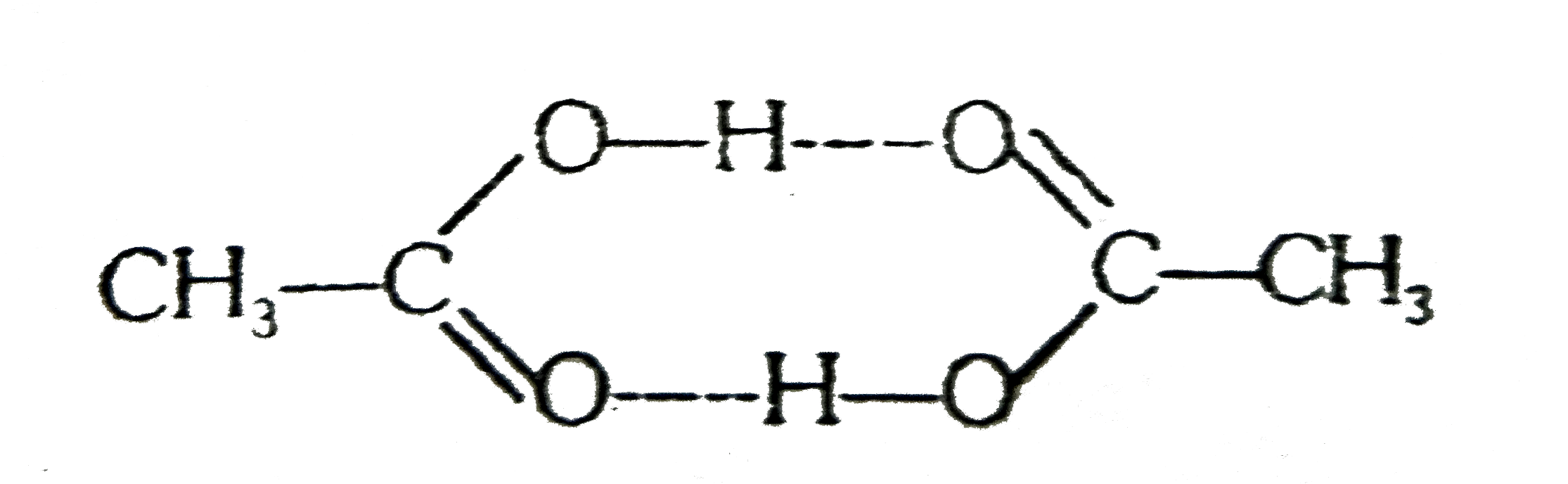
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-SOLUTIONS-EXERCISE-05 [A]
- A5% solution of cane sugar (molar mass =342)is isotonic with 1%of a so...
Text Solution
|
- The molality of a urea solution in which 0.0100 g of urea, [(NH(2))...
Text Solution
|
- K(f) for waer is 1.86 K kg mol^(-1). If your automobile radiator holds...
Text Solution
|
- The freezing point of a 1.00 m aqueous solution of HF is found to be -...
Text Solution
|
- Two liquids A and B form an ideal solution. At 300 K, the vapour press...
Text Solution
|
- A solution containing 0.85 g of ZnCI(2) in 125.0g of water freezes at ...
Text Solution
|
- A solution containing 0.85 g of ZnCI(2) in 125.0g of water freezes at ...
Text Solution
|
- 12g of a nonvolatile solute dissolved in 108g of water produces the re...
Text Solution
|
- A molecule M associates in a given solvent according to the equation M...
Text Solution
|
- Vapour pressure of pure benzene is 119 torr and that of toluene is 37....
Text Solution
|
- How maby grams of methyl alcohol should be added to 10 litre tank of w...
Text Solution
|
- Consider separate solution of 0.500M C(2)H(5)OH(aq), 0.100M Mg(3)(PO(4...
Text Solution
|
- For an ideal solution of two components A and B, which of the followin...
Text Solution
|
- The observed osmotic pressure for a 0.10M solution of Fe(NH(4))(2)(SO(...
Text Solution
|
- The vapor pressure of acetone at 20^(@)C is 185 torr. When 1.2 g of a ...
Text Solution
|
- A solution at 20^(@)C is composed of 1.5 mol of benzene and 3.5 mol o...
Text Solution
|
- Determination of the molar mass of acetic acid in benzene using freezi...
Text Solution
|
- 18g glucose (C(6)H(12)O(6)) is added to 178.2g water. The vapour press...
Text Solution
|
- The solubility of N(2) in water at 300K and 500 torr partial pressure ...
Text Solution
|
- An aqueous solution of a salt MX(2) at certain temperature has a van'f...
Text Solution
|