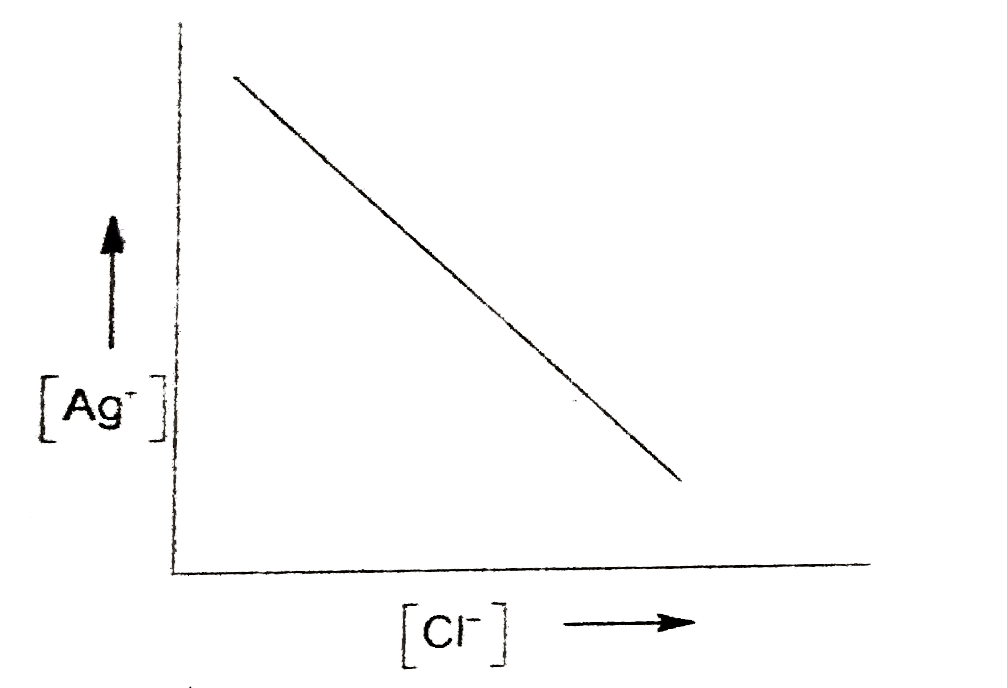
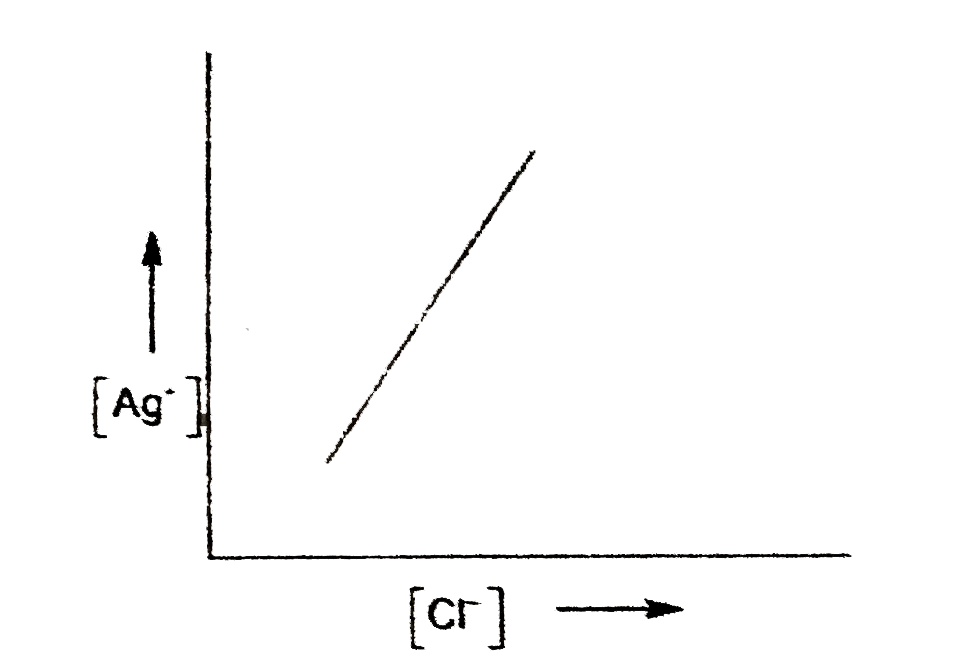
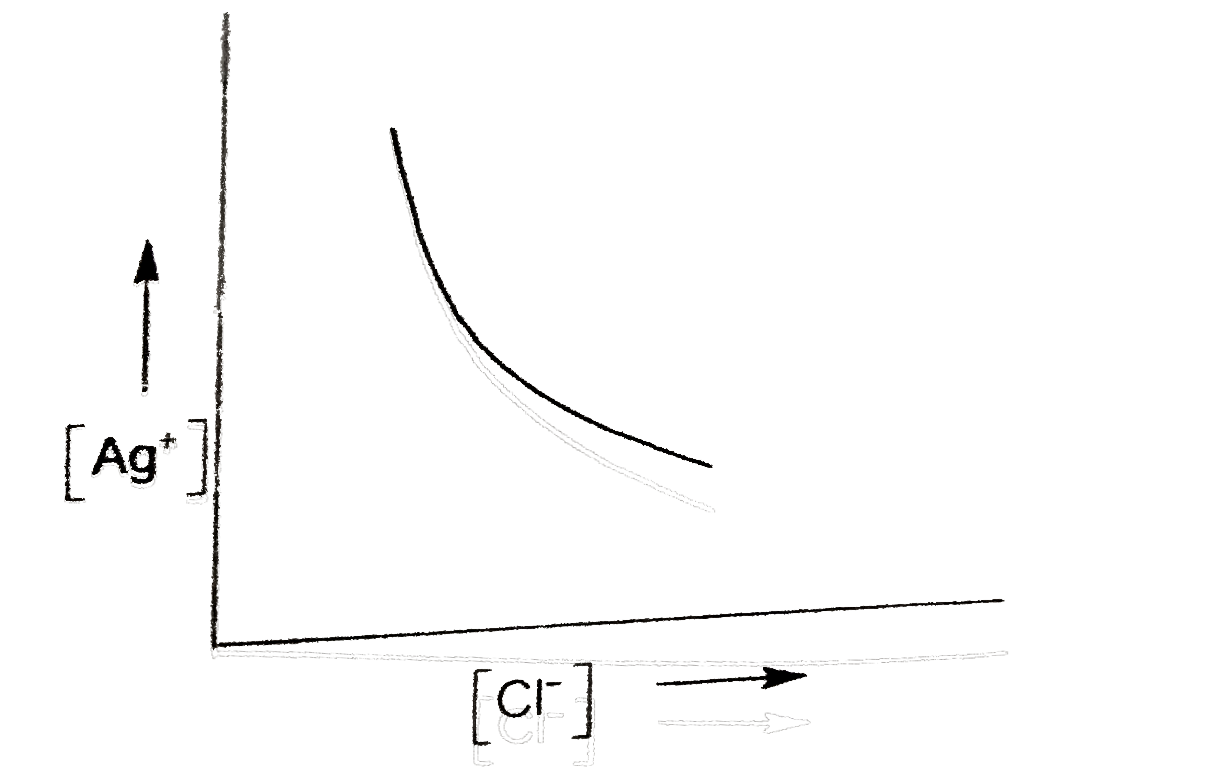
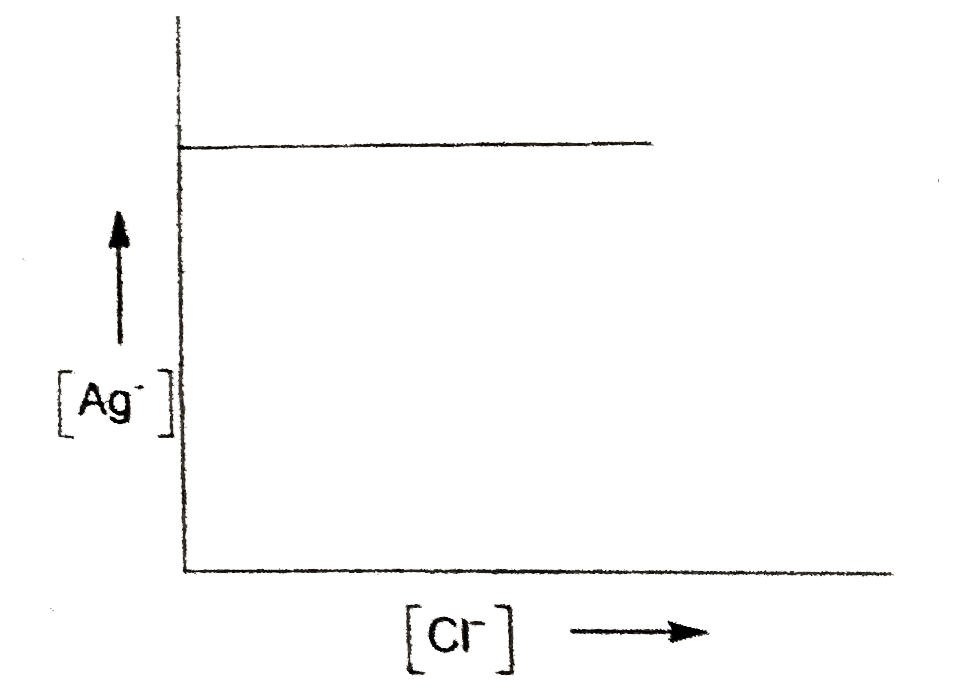
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
FIITJEE-TEST PAPERS-CHEMISTRY
- The equilibrium constant K(P) for the reaction N(2)O(4)(g) hArr 2NO(2)...
Text Solution
|
- What is the hydronium ion concentration of a 0.25 M HA solution (K(a)=...
Text Solution
|
- In a saturated solution of AgCl, NaCl is added gradually the concentra...
Text Solution
|
- A solution is 0.01 M Kl and 0.1 M KCl. If solid AgNO(3) is added to th...
Text Solution
|
- Solubility of AgCl in 0.2 M NaCl is x and that in 0.1 M AgNO(3) is y. ...
Text Solution
|
- The incorrect order of bond angle
Text Solution
|
- All fluorine atoms are in same plane in
Text Solution
|
- Consider a P(y) orbital of an atom and identify correct statement
Text Solution
|
- The set of d-orbitlas which do not contain any d-orbital which is invo...
Text Solution
|
- Select the correct order of hydration energy of ions?
Text Solution
|
- Decomposition temperature of CaCO(3)(s) is approximately 900^(@)C. Whi...
Text Solution
|
- Select the correct order of basic strength?
Text Solution
|
- Be and Mg have zero value of electron affinity, because
Text Solution
|
- Out of N, O, Ne, Na, Na^(+) select the species which have maximum and ...
Text Solution
|
- The correct ionic radii order is
Text Solution
|
- Boron compound behave as Lewis acid because of
Text Solution
|
- H(3)BO(3) is.
Text Solution
|
- Graphite is soft solid lubricant extreamly to melt. The reason for thi...
Text Solution
|
- The reaction 5H(2)O(2)+XClO(2)+2OH^(-) rarr XCl^(-)+YO(2)+6H(2)O i...
Text Solution
|
- Consider the following reaction x MnO(4)^(-) + C(2)O(4)^(2-) + zH^(+...
Text Solution
|