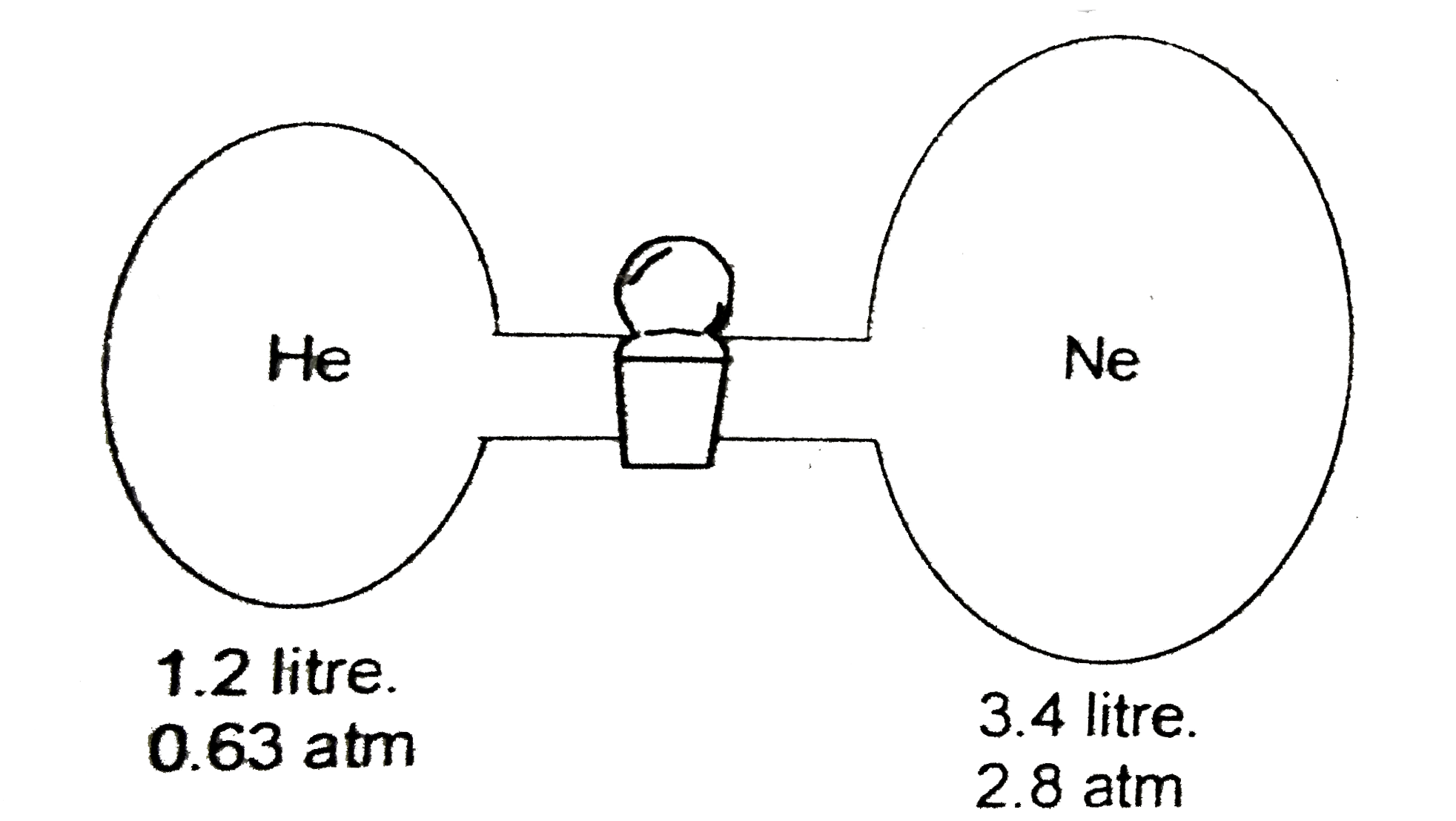
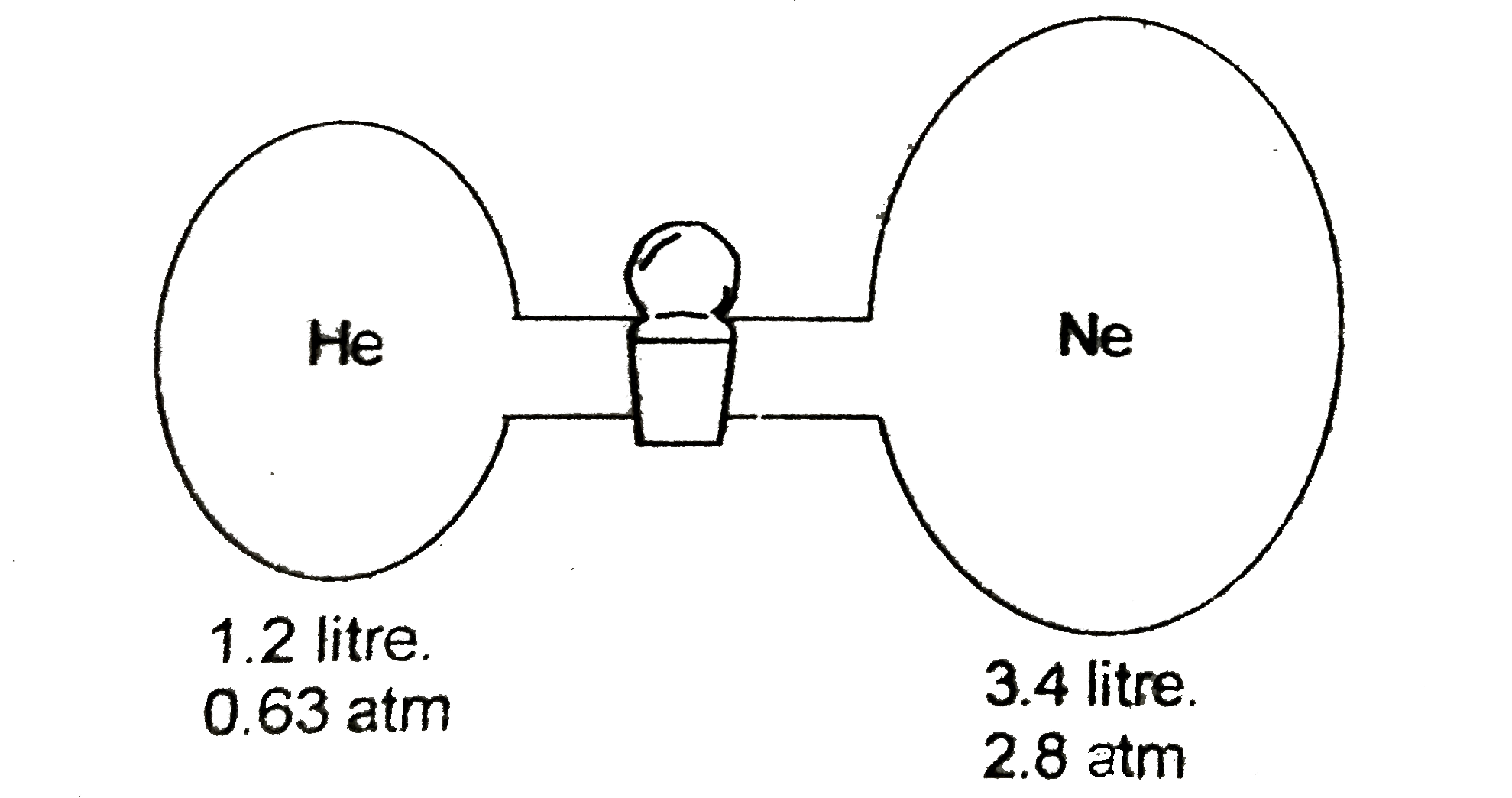
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
FIITJEE-TEST PAPERS-CHEMISTRY
- For a reversible adiabatic ideal gas expansion (dp)/(p) is equal to
Text Solution
|
- A freshly prepared Fe(OH)(3) precipitate is peptized by adding FeCl(3)...
Text Solution
|
- Consider the following apparatus. Calculate the partical pressure of H...
Text Solution
|
- On adding Kl to a metal salt solution, no precipitate was observed but...
Text Solution
|
- Which of the following is true in respect of chemical adsorption?
Text Solution
|
- Hydrazine reacts with KIO(3) in presence of HCl as : N(2)H(4)+IO(3)^...
Text Solution
|
- Which of the following statement is false?
Text Solution
|
- Consider the following reaction: H(2)O(l)toH(2)O(g)" "DeltaH(1)=44...
Text Solution
|
- Smog is essentially caused by the presence of
Text Solution
|
- Which of the following is not tranquilizer?
Text Solution
|
- Which of the following will not be oxidised by O(3)?
Text Solution
|
- Which of the following process is used in the extractive metallurgy of...
Text Solution
|
- Which of the following compound is not an actacid?
Text Solution
|
- The gas leaked from a stronge tank of the Union Carbide plant in Bhopa...
Text Solution
|
- 36 mL of pure water takes 100 sec to evaporate from a vessel when a he...
Text Solution
|
- 108 g fairly concentrated solution of AgNO(3) is electrolysed by using...
Text Solution
|
- The geometry, hybridization and magnetic moment of [Ni(CO)(4)] are …. ...
Text Solution
|
- X=amount of gas adsorbed P=Pressure T=Temperature L=Physisorptio...
Text Solution
|
- The correct statement regarding the third orbit of hydrgen atom is
Text Solution
|
- What is the unit of rate constant of the reaction for which the above ...
Text Solution
|