A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
FIITJEE-TEST PAPERS-CHEMISTRY
- 108 g fairly concentrated solution of AgNO(3) is electrolysed by using...
Text Solution
|
- The geometry, hybridization and magnetic moment of [Ni(CO)(4)] are …. ...
Text Solution
|
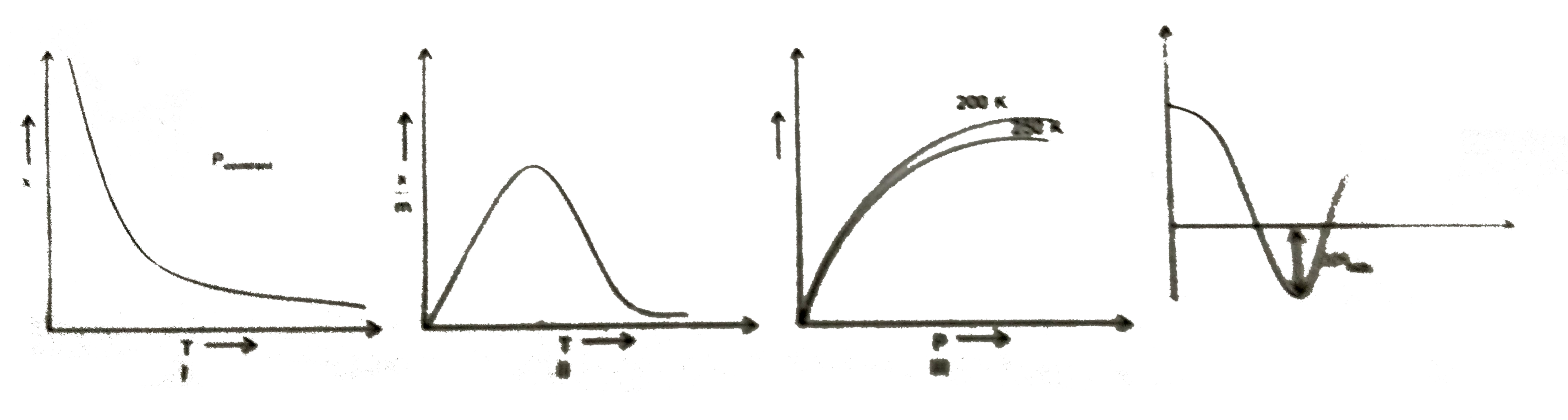
- X=amount of gas adsorbed P=Pressure T=Temperature L=Physisorptio...
Text Solution
|
- The correct statement regarding the third orbit of hydrgen atom is
Text Solution
|
- What is the unit of rate constant of the reaction for which the above ...
Text Solution
|
- SO(3)(g)+CO(g)hArrSO(2)(g)+CO(2)(g) Which of the following activity ...
Text Solution
|
- 200 mL of 0.4M solution of CH(3)COONa is mixed with 400 mL of 0.2 M so...
Text Solution
|
- Which of the following bond angle is not observed in PCl(5) molecule i...
Text Solution
|
- Which of the following produces a coloured gas on heating?
Text Solution
|
- Which of the following type of compound is mixed with detergent powder...
Text Solution
|
- In which of the following compound, chlorinie exerts the maximum numbe...
Text Solution
|
- The soda extract of which of the following commpound gives the test of...
Text Solution
|
- Which of the following linkage is present in 'Teflon'?
Text Solution
|
- Which of the following reaction does not produce H(3)PO(4)?
Text Solution
|
- Which of the following reaction produce Cl(2) gas?
Text Solution
|
- Which of the following is a colourless complex ion?
Text Solution
|
- Which of the following metallurgical process is not needed in order to...
Text Solution
|
- Which of the following salt can increase te boiling point of water by ...
Text Solution
|
- Which of the following ion has the highest coagulation power for the s...
Text Solution
|
- A hydrogen electrode is prepared by using a sample of HCl solution wit...
Text Solution
|