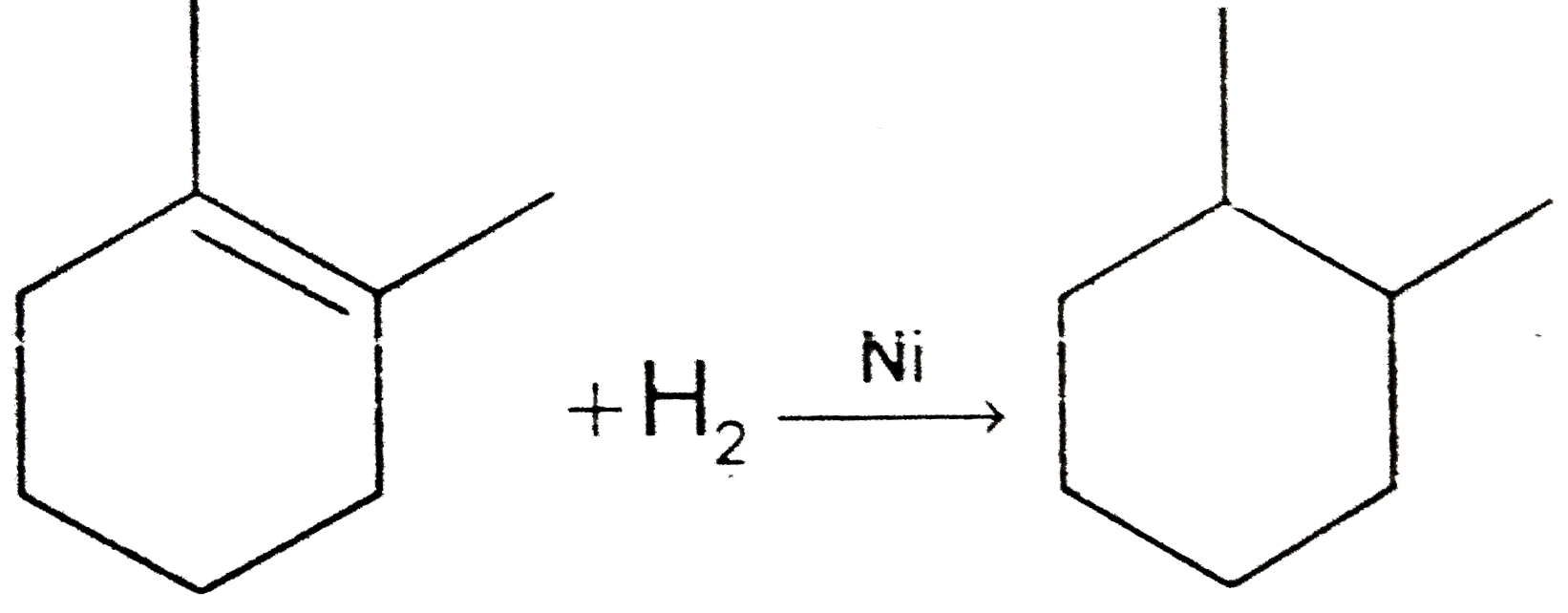
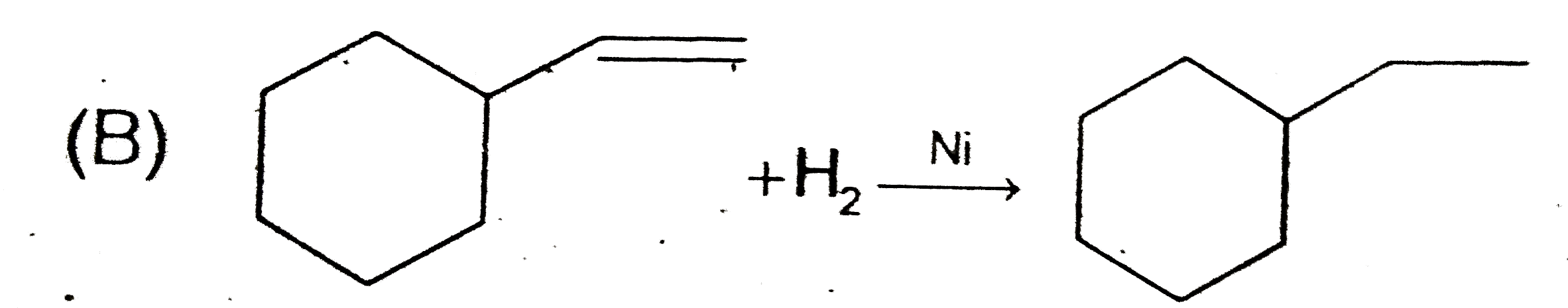
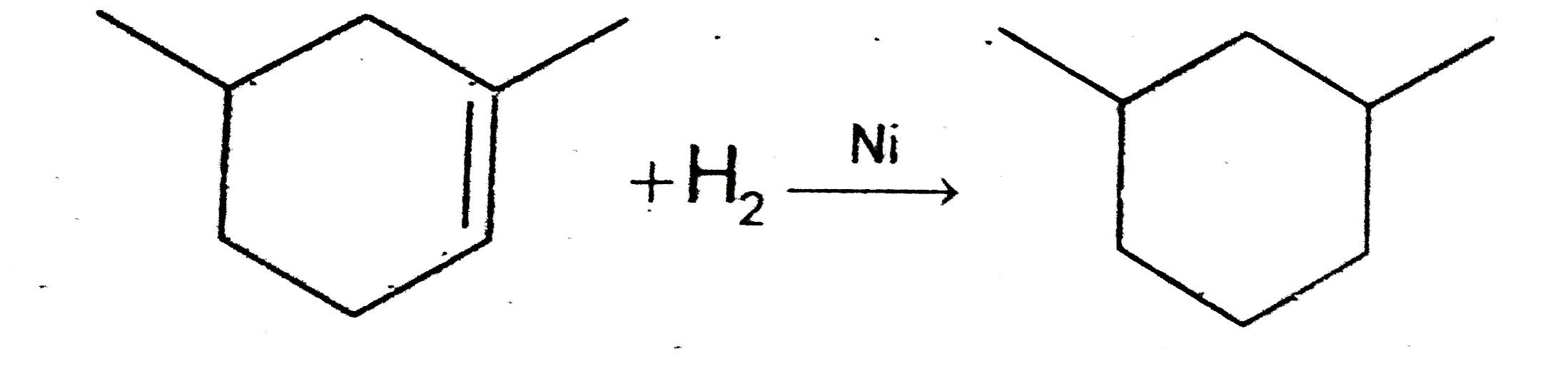
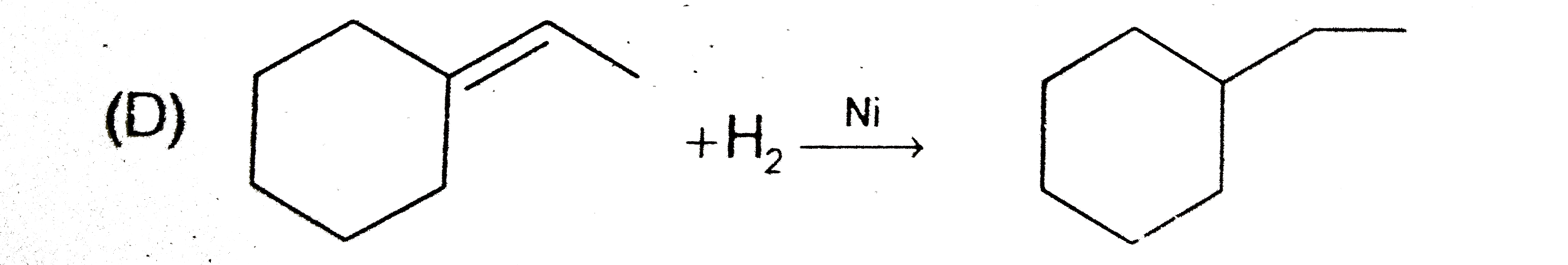A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
FIITJEE-TEST PAPERS-CHEMISTRY
- The half-life of a chemical reaction does not change by either increas...
Text Solution
|
- K(sp) of AgCl in water at 25^(@)C is 1.8xx10^(-10). If 10^(-5) mole of...
Text Solution
|
- Which of the following reaction liberates maximum amount of heat energ...
Text Solution
|
- A diene on reductive ozonolysis produces two moles of ethanal and one ...
Text Solution
|
- how much work in kJ mol^(-1) unit is done by reversible and isothermal...
Text Solution
|
- In which of the following alcohols C-O bond breaks (heterolytically) w...
Text Solution
|
- Which of the following has the most acidic hydrogen ?
Text Solution
|
- Dehydration of 1-butanol gives 2-butane as a major product, by which o...
Text Solution
|
- In which of the following crystals alternate tetrahedral voids are occ...
Text Solution
|
- Molar heat capacity of an ideal gas at constant volume is given by C(V...
Text Solution
|
- BCl(3)+H(2)+Coverset(1700-1800^(@)C)toProduct(s) The product(s) of a...
Text Solution
|
- Atomic radii of Ti, Zn and Hf vary.
Text Solution
|
- C(6)H(5)CHO+CH(3)COOCOCH(3)underset(Delta)overset(CH(3)COONa)to produc...
Text Solution
|
- the product of above reaction is
Text Solution
|
- Which forms aromatic compound on heating?
Text Solution
|
- The shape of [Co(NH(3))(6)]^(3+) is
Text Solution
|
- AgNO(3) solution will not give white precipitate with
Text Solution
|
- Which of the following two reagents form silicates when react with sil...
Text Solution
|
- In which combinations all the ligands are ambient in nature?
Text Solution
|
- Which of the following statements about photochemical smog is wrong ?
Text Solution
|