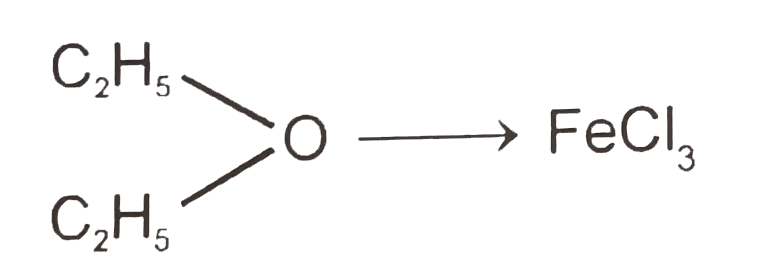
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
D BLOCK ELEMENTS
RESONANCE|Exercise EXERCISE-I PART-III|15 VideosD BLOCK ELEMENTS
RESONANCE|Exercise EXERCISE-2 PART-I|27 VideosD BLOCK ELEMENTS
RESONANCE|Exercise EXERCISE-I PART-I|40 VideosD & F BLOCK ELEMENTS
RESONANCE|Exercise INORGANIC CHEMISTRY(d & f- Block Elments)|40 VideosDPP
RESONANCE|Exercise QUESTIONS|223 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-D BLOCK ELEMENTS-EXERCISE-I PART-II
- CuSO(4) solution + lime is called:
Text Solution
|
- The developer used in photography is an alkaline solution of:
Text Solution
|
- When acidified solution of K(2)Cr(2)O(7) is shaken with aqeous solutio...
Text Solution
|
- FeCl(3) dissolves in:
Text Solution
|
- Which of the following compounds is used as the startingn material for...
Text Solution
|
- Cr)(3) dissolved in aqueous NaOH to give:
Text Solution
|
- The final product obtained for the following reaction is: KMnO(4)("ex...
Text Solution
|
- When AgNo(3)(aq) reacts with excess of iodine, we get:
Text Solution
|
- ZnO+CoOundersetto(Delta)X,Product X colour is:
Text Solution
|
- The compound that gets oxidised even on exposure to atmosphere is:
Text Solution
|
- Select correct statement(s).
Text Solution
|
- The f-block elements of the periodic table contains those element in w...
Text Solution
|
- Among the lanthanides the one obtained by synthetic method is
Text Solution
|
- The most common lanthanoide is :
Text Solution
|
- Across the lanthanide series , the basicity of the lanthanoide hydroxi...
Text Solution
|
- The +3 ion of which one of the following has half filled 4f subshell?
Text Solution
|
- Actinides
Text Solution
|
- The lanthanide contraction is responsible for the fact that
Text Solution
|
- Lanthanides and actinides resemble in
Text Solution
|
- The speration of lanthanides by ion exchanges method is based on
Text Solution
|