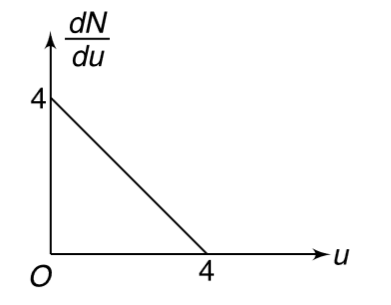
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A hypothetical gas sample has its molecular speed distribution graph a...
Text Solution
|
- The root mean square speed of gas molecules
Text Solution
|
- Graph shows a hypothetical speed distribution for a sample of N gas pa...
Text Solution
|
- A hypothetical gas sample has its molecular speed distribution graph a...
Text Solution
|
- The speed distribution of molecules in a sample of a gas is shown in t...
Text Solution
|
- Graph shows a hypothetical speed distribution for a sample of N gas pa...
Text Solution
|
- किसी गैस के 5 अणुओं की चाल (स्वच्छ मात्रक में) 2,3,4,5,6 है । इन अणुओं...
Text Solution
|
- The root mean square speed of the molecules of a gas is
Text Solution
|
- In the distribution of molecular speeds of a gas is as per the figure ...
Text Solution
|