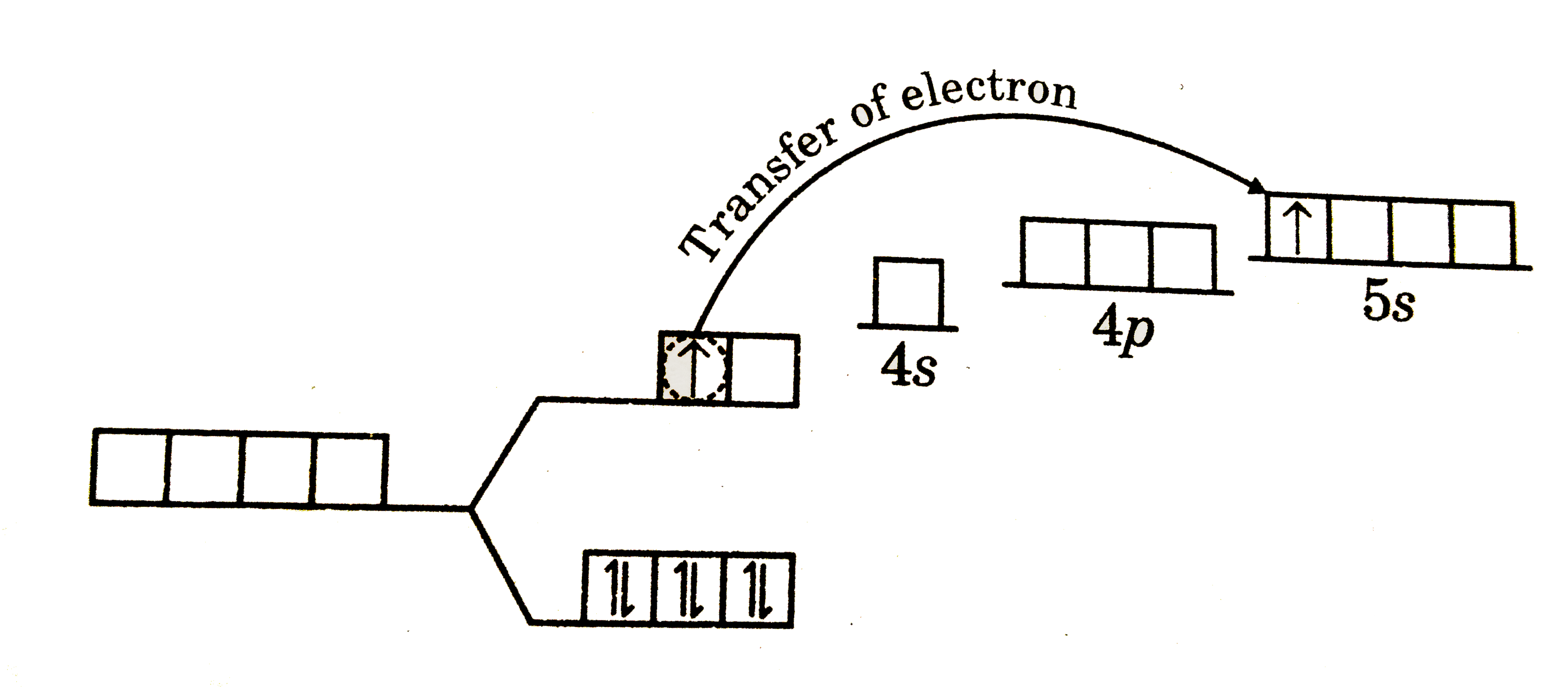
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
OP TANDON|Exercise E. Stability, Colour and Magnetic Properties of Complexes|60 VideosCOORDINATION COMPOUNDS
OP TANDON|Exercise F.Isomerism in Coordination Compounds|120 VideosCOORDINATION COMPOUNDS
OP TANDON|Exercise C. Werners Theory, EAN Rule|63 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
OP TANDON|Exercise INTEGER|2 VideosELECTROCHEMISTRY
OP TANDON|Exercise Matrix-Matching Type Question|3 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-COORDINATION COMPOUNDS-D.VBT, CFT, Hybridisation
- Which of the following option is correct regarding [Fe(H(2)O)(5)NO]SO(...
Text Solution
|
- The colourless and paramagnetic compound is :
Text Solution
|
- Which of the following is most easily oxidised ?
Text Solution
|
- The geometry of FeCl(4)^(2-), FeCl(2)(PPh(3))(2) and AuCl(4)^(-) would...
Text Solution
|
- It is an experiment fact that Cs(2)[CuCl(4)] is orange coloured but NH...
Text Solution
|
- All the following complexes show a decrease in their weights when plac...
Text Solution
|
- It is an experimental fact that : DMG+Ni(II)"salt"+NH(4)OH to "Red p...
Text Solution
|
- The correct order for the CFSE (numerical value) for the following com...
Text Solution
|
- A square planar complex is formed by hybridisation of which atomic orb...
Text Solution
|
- The CFSE for octahedral [CoCl(6)]^(4-) complex is 18000 cm^(-1). Then,...
Text Solution
|
- In nitroprusside ion the iron and NO exist as Fe^(II) and NO^(+) rathe...
Text Solution
|
- The complex ions [Fe(CN)(6)]^(3-) and [Fe(CN)(6)]^(4-) :
Text Solution
|
- Which of the following statement is correct for complex [Cr(NH(3))(CN)...
Text Solution
|
- Which of the following complex involves d^(2)sp^(3) hybridization ?
Text Solution
|
- Which is true for [Ni(en)(2)]^(2+) ? (Atomic number of nickel is 28)
Text Solution
|
- The crystal field-splitting for Cr^(3+) ion in octahedral field change...
Text Solution
|
- Arrange the following in order of decreasing number of unpaired electr...
Text Solution
|
- Which of the following statement is false ?
Text Solution
|
- The [Fe(CN)(6)]^(3-) complex ion :
Text Solution
|
- Consider the following statements : (P) [Mn(H(2)O)(4)]SO(4) is param...
Text Solution
|