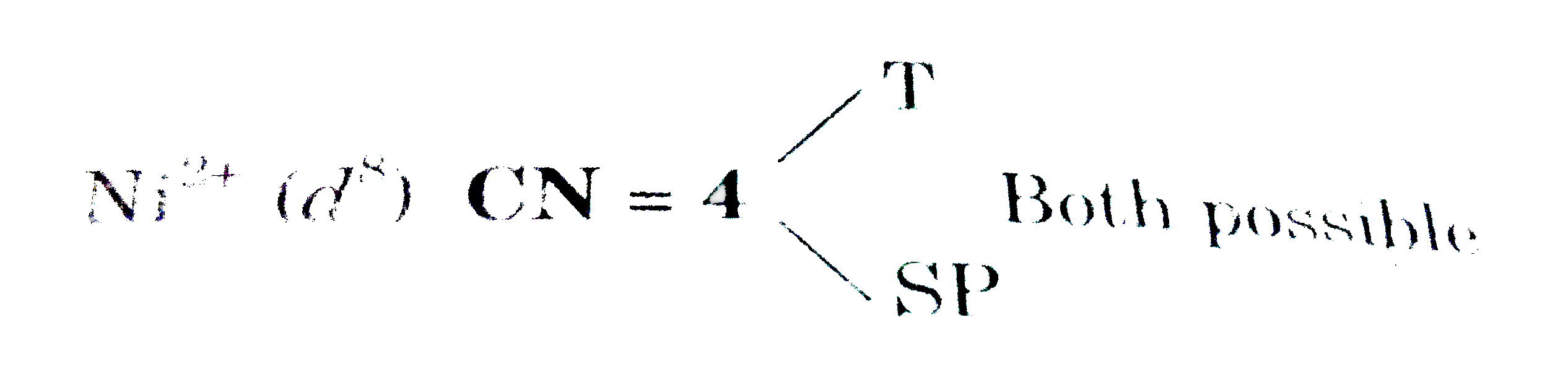A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
OP TANDON|Exercise COMPREHENSION 1|3 VideosCOORDINATION COMPOUNDS
OP TANDON|Exercise COMPREHENSION 2|3 VideosCOORDINATION COMPOUNDS
OP TANDON|Exercise REASONING TYPE|59 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
OP TANDON|Exercise INTEGER|2 VideosELECTROCHEMISTRY
OP TANDON|Exercise Matrix-Matching Type Question|3 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-COORDINATION COMPOUNDS-MULTIPLE OBJECTIVE TYPE QUESTION
- Select incorrect statement about the complex : cis-[Rh(H)(CO)(Pme(3))(...
Text Solution
|
- Select the correct statements about Ma(2)bcde
Text Solution
|
- In which of the following complex(s) spin only magnetic moment is inde...
Text Solution
|
- Which of the following species is/are coloured ?
Text Solution
|
- Which of the following complexes is/are tetrahedral ?
Text Solution
|
- Which of the following molecules is/are planar ?
Text Solution
|
- VCl(3)(THF)(3)overset(Mg,ZnI(2))underset(CO,Delta,pressure) to Na[V(CO...
Text Solution
|
- Which of the following names is/are correct for the compound Na[CoCl(2...
Text Solution
|
- Identify pair of neutral co-ordination entity amongst the following ...
Text Solution
|
- EDTA^(4-) is an important ligand. Which statement about this ligand is...
Text Solution
|
- Choose the correct statement about Metal Carbonyls :
Text Solution
|
- Choose the correct statement(s) :
Text Solution
|
- CoCl(3).6NH(3) can form 4 different types of complexes (According to W...
Text Solution
|
- Which of the following complexes are capable of showing geometrical is...
Text Solution
|
- Choose the correct statement(s).
Text Solution
|
- Choose the incorrect statement(s).
Text Solution
|
- Werner's theory could not explain :
Text Solution
|
- In which of the ion pairs, magnetic property depends on the strngth of...
Text Solution
|
- Which of the following ions can form paramagnetic as well as diamagnet...
Text Solution
|
- Find the number of unpaired electrons in each case [Mn(CN)(6)]^(3-)=...
Text Solution
|