A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
KINETIC THEORY
AAKASH INSTITUTE|Exercise Assignment (Section-B) Objective type questions (One option is correct)|9 VideosKINETIC THEORY
AAKASH INSTITUTE|Exercise Assignment (Section-C) Objective type questions (More than one option are correct)|9 VideosKINETIC THEORY
AAKASH INSTITUTE|Exercise Try Yourself|43 VideosGRAVITATION
AAKASH INSTITUTE|Exercise ASSIGNMENT SECTION - D (ASSERTION-REASON TYPE QUESTIONS)|16 VideosLAWS OF MOTION
AAKASH INSTITUTE|Exercise Assignment (SECTION-D) (Assertion-Reason Type Questions)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-KINETIC THEORY-Assignment (Section-A) Objective type questions (One option is correct)
- For Boyle's law to be hold good, the gas should be
Text Solution
|
- Boyle's law is applicable for an
Text Solution
|
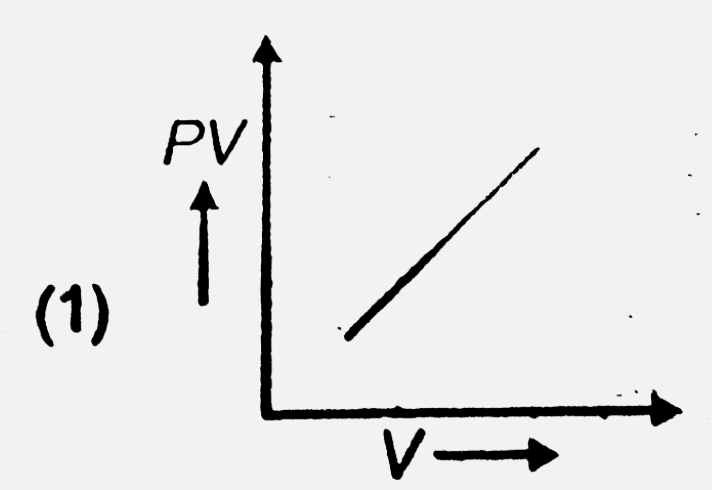
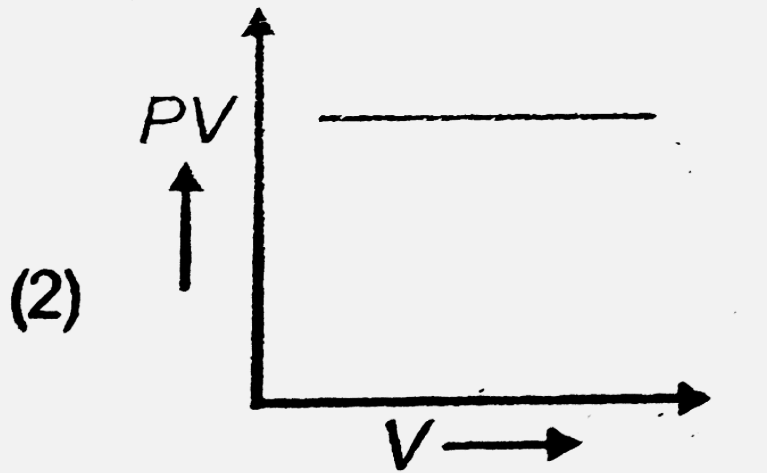
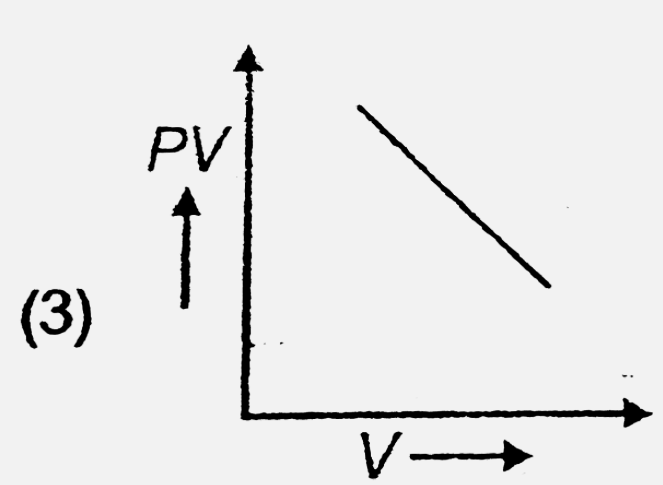
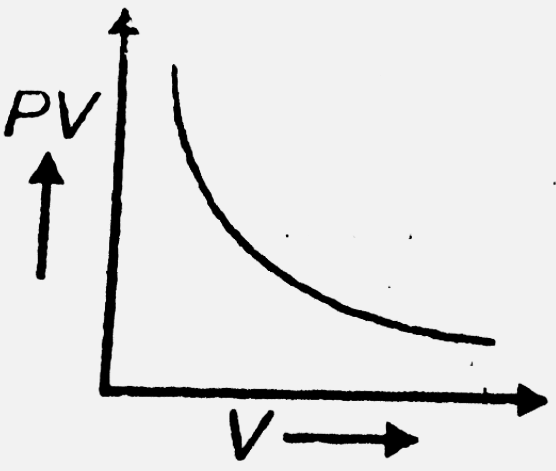
- The variation of PV with V of fixed mass of an ideal gas at constant t...
Text Solution
|
- Two identical cylinders contains helium at 1.5 atm and argon at 1 atm ...
Text Solution
|
- Two gases A and B having the same temperature T, same pressure P and s...
Text Solution
|
- A perfect gas at 27^(@)C is heated at constant pressure so as to tripl...
Text Solution
|
- Which of the following methods will enable the volume of an ideal gas ...
Text Solution
|
- Four molecules of a gas have speeds 2, 4, 6 and 8 kms^(-1) respectivel...
Text Solution
|
- The rms speed of a gas molecule is
Text Solution
|
- The temperature of a gas is raised while its volume remains constant, ...
Text Solution
|
- If the pressure in a closed vessel is reduced by drawing out some gas,...
Text Solution
|
- The pressure P, Volume V and temperature T of a gas in the jar A and t...
Text Solution
|
- A container has N molecules at absolute temperature T. If the number o...
Text Solution
|
- 2000 small balls, each weighing 1 g, strike one square cm of area per ...
Text Solution
|
- Some gas at 100 K is enclosed in a container. Now the container is pla...
Text Solution
|
- During experiment an ideal gas is found to obey an additional law VP^(...
Text Solution
|
- Two gases A and B having the same pressure P, volume V and temperature...
Text Solution
|
- A gas is heated through 2^(@)C in a closed vessel. Its pressure is inc...
Text Solution
|
- A balloon contains 1500 m^(3) of helium at 27^(@)C and 4 atmospheric p...
Text Solution
|
- Gas at a pressure P(0) in contained as a vessel. If the masses of all ...
Text Solution
|