Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the cyclic process abca performed on a sample of 2.0 mole of ...
Text Solution
|
- consider the cyclic process ABCA on a sample of 2.0 mol of an ideal ga...
Text Solution
|
- Consider the cyclic process ABCA, shown in, performed on a sample of ...
Text Solution
|
- An ideal monoatomic gas undergoes a cyclic process ABCA as shown in th...
Text Solution
|
- A cyclic process performed on one mole of an ideal gas. A total 1000 J...
Text Solution
|
- In figure, a sample of 3 moles of an ideal gas is undergoing through a...
Text Solution
|
- Consider the cyclic process abca performed on a sample of 2.0 mole of ...
Text Solution
|
- A sample of gas is going through cyclic process, work done by gas is
Text Solution
|
- consider the cyclic process ABCA on a sample of 2.0 mol of an ideal ga...
Text Solution
|
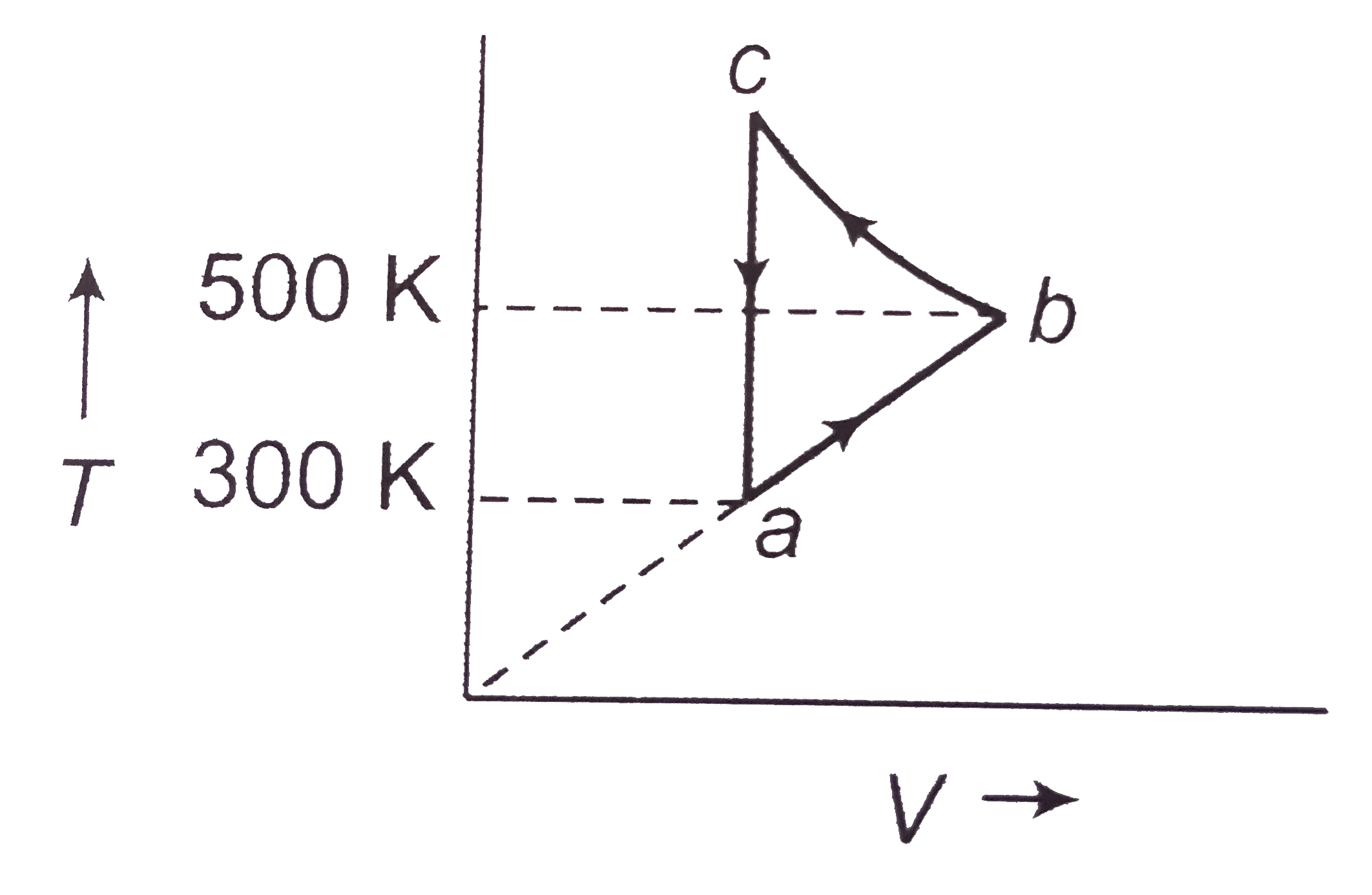
 Consider the cyclic process abca performed on a sample of 2.0 mole of an ideal gas. A total of 1000 cal of heat is withdrawn from the sample in the process. Find the work done by the gas during the past bc.
Consider the cyclic process abca performed on a sample of 2.0 mole of an ideal gas. A total of 1000 cal of heat is withdrawn from the sample in the process. Find the work done by the gas during the past bc.