Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the following cyclic abcda performed on 2 mole of an ideal ga...
Text Solution
|
- shows the variation in the internal energy U with the volume V of 2.0 ...
Text Solution
|
- Fig. shows the variation of internal energy (U) with the pressure (P) ...
Text Solution
|
- Consider the following cyclic abcda performed on 2 mole of an ideal ga...
Text Solution
|
- A sample of ideal gas is taken through the cyclic process shown in the...
Text Solution
|
- In a thermodynamic process two moles of a monatomic ideal gas obeys PV...
Text Solution
|
- One mole of an ideal monoatomic gas undergoes a cyclic process, as sho...
Text Solution
|
- One mole of an ideal monoatomic gas undergoes a cyclic process, as sho...
Text Solution
|
- एकपरमाणुक आदर्श गैस के 1 मोल को चित्र में दिखाए गए चक्रीय प्रक्रम ABCA...
Text Solution
|
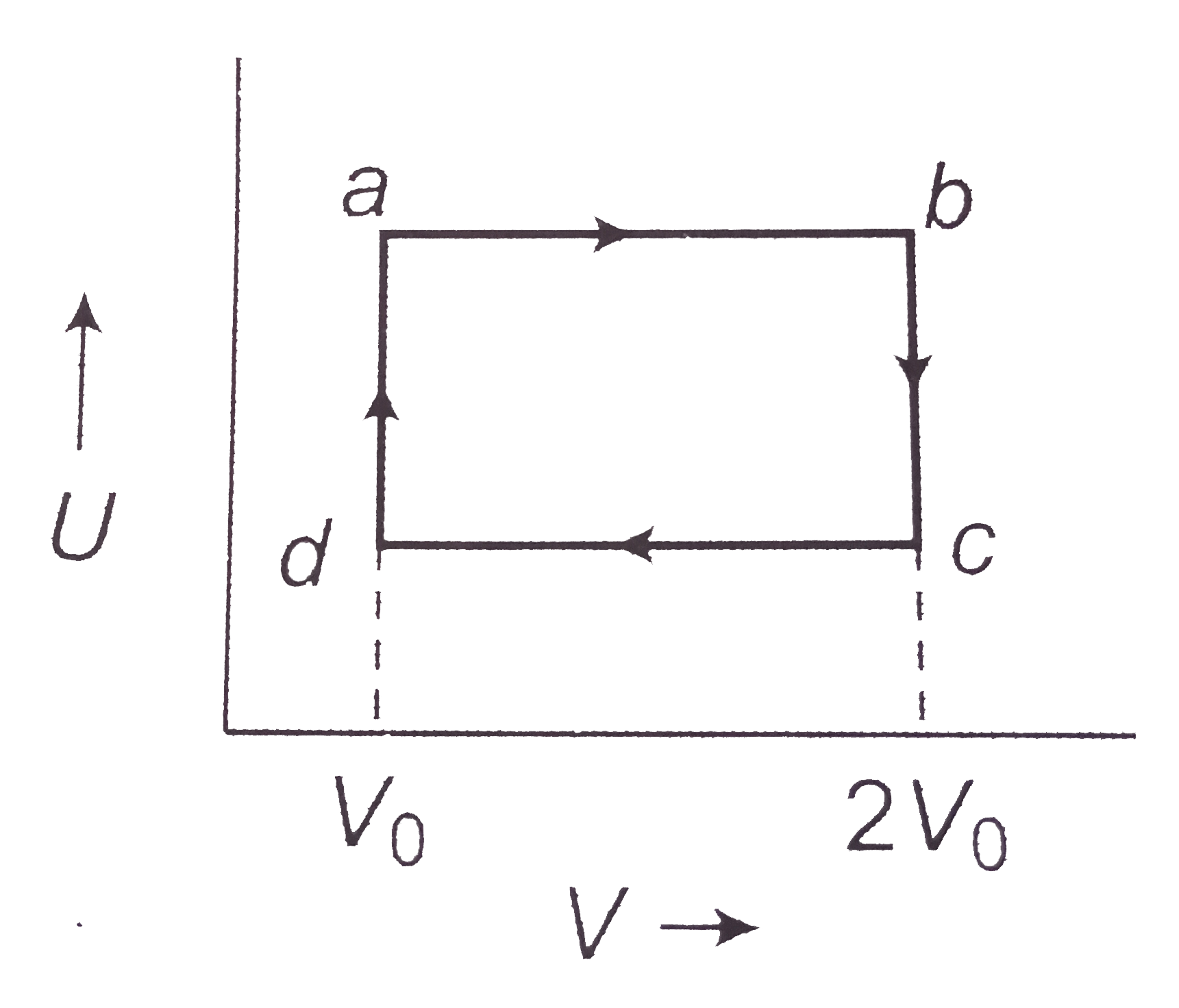 Consider the following cyclic abcda performed on 2 mole of an ideal gas. The temperature of the gas at b and c are 400 k and 300 k respectively. Calculated the heat absorbed by the gas during the process in calories.
Consider the following cyclic abcda performed on 2 mole of an ideal gas. The temperature of the gas at b and c are 400 k and 300 k respectively. Calculated the heat absorbed by the gas during the process in calories.