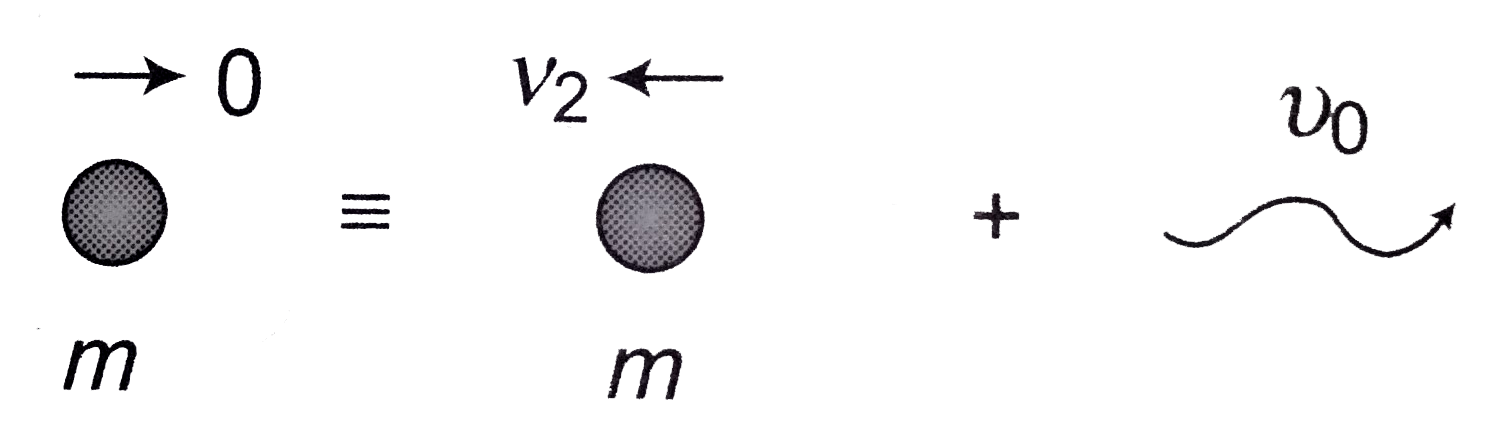Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CP SINGH-BOHR THEORY-Exercises
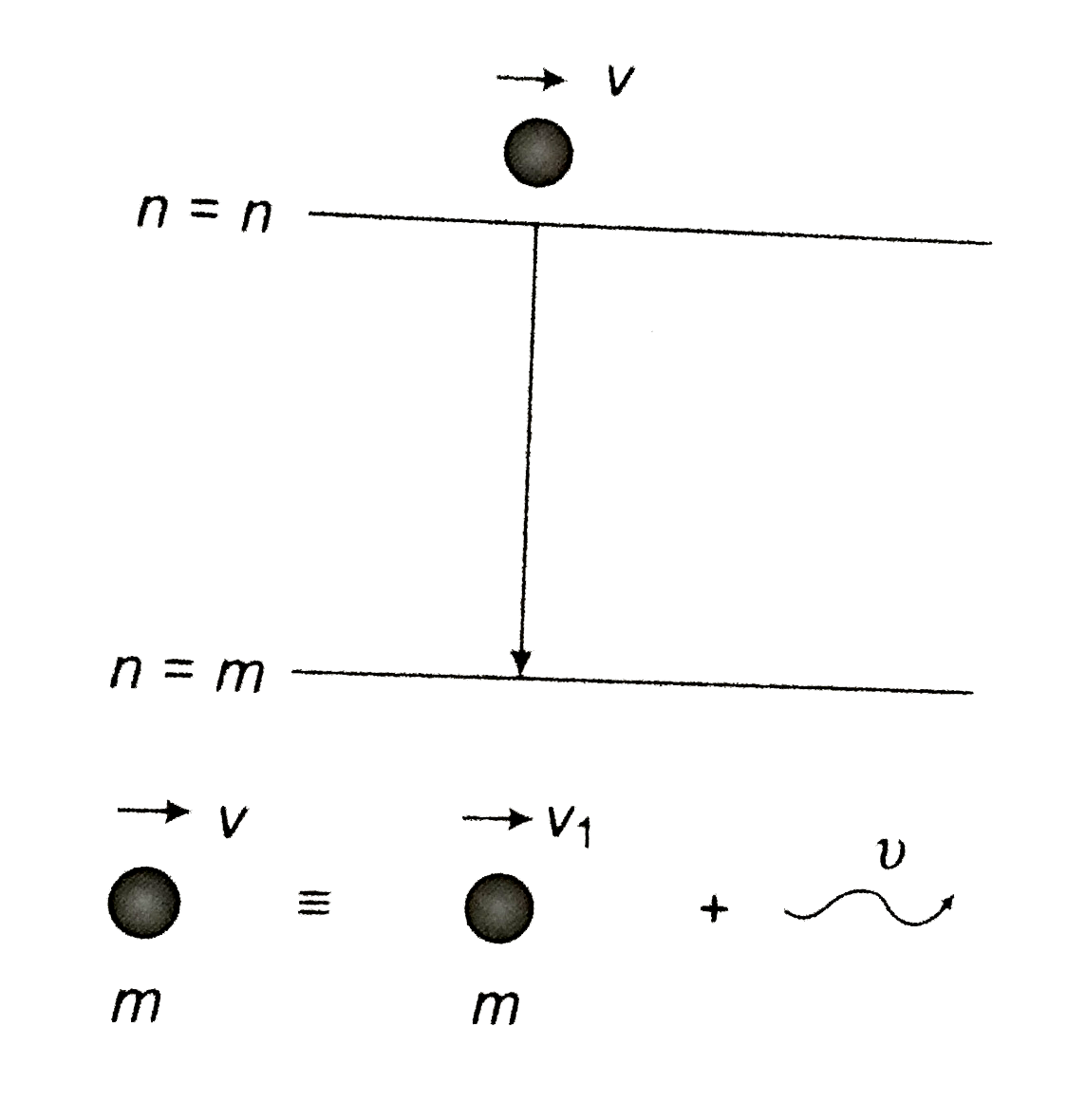
- Consider an excited hydrogen atom in state n moving with a velocity v(...
Text Solution
|
- A beam of fast moving alpha particles were directed towards a thin fil...
Text Solution
|
- In a Rutherford scattering experiment when a projectile of change Z(1)...
Text Solution
|
- An alpha-particle of 5MeV energy strikes with a nucleus of uranium at ...
Text Solution
|
- An alpha nucleus of energy (1)/(2)m nu^(2) bombards a heavy nucleus o...
Text Solution
|
- In Rutherford scattering experiment, what will b ethe correct angle fo...
Text Solution
|
- Which of the following is correct regarding Bohr's theory for hydrogen...
Text Solution
|
- In the Bohr model of the atom (i) the radius of the n^(th) orbit is ...
Text Solution
|
- The minimum orbital angular momentum of the electron in a hydrogen ato...
Text Solution
|
- Which of the following parameters are the same for all hydrogen like a...
Text Solution
|
- As one considers orbits with higher value of n is a hydrogen atom, the...
Text Solution
|
- The enrgy of an atom (or ion) in the ground state is - 54.4 eV .If ma...
Text Solution
|
- The angular speed of the electron in the n^(th) Bohr orbit of the hydr...
Text Solution
|
- According to Bohr's theory, the ratio of the times taken by the electr...
Text Solution
|
- The ratio of the binding energies of the hydrogen atom in the first an...
Text Solution
|
- The radius of the shortest orbit in a one electron system is 18 pm it...
Text Solution
|
- Which of the following curve may represent the speed of the electron i...
Text Solution
|
- In hydrogen atom, if the difference in the energy of the electron in n...
Text Solution
|
- An orbit electron in the ground state of hydrogen has an angular momen...
Text Solution
|
- In Bohr's model of hydrogen atom, let PE represents potential energy a...
Text Solution
|
- The ratio of the speed of the electrons in the ground state of hydroge...
Text Solution
|