A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CP SINGH-BOHR THEORY-Exercises
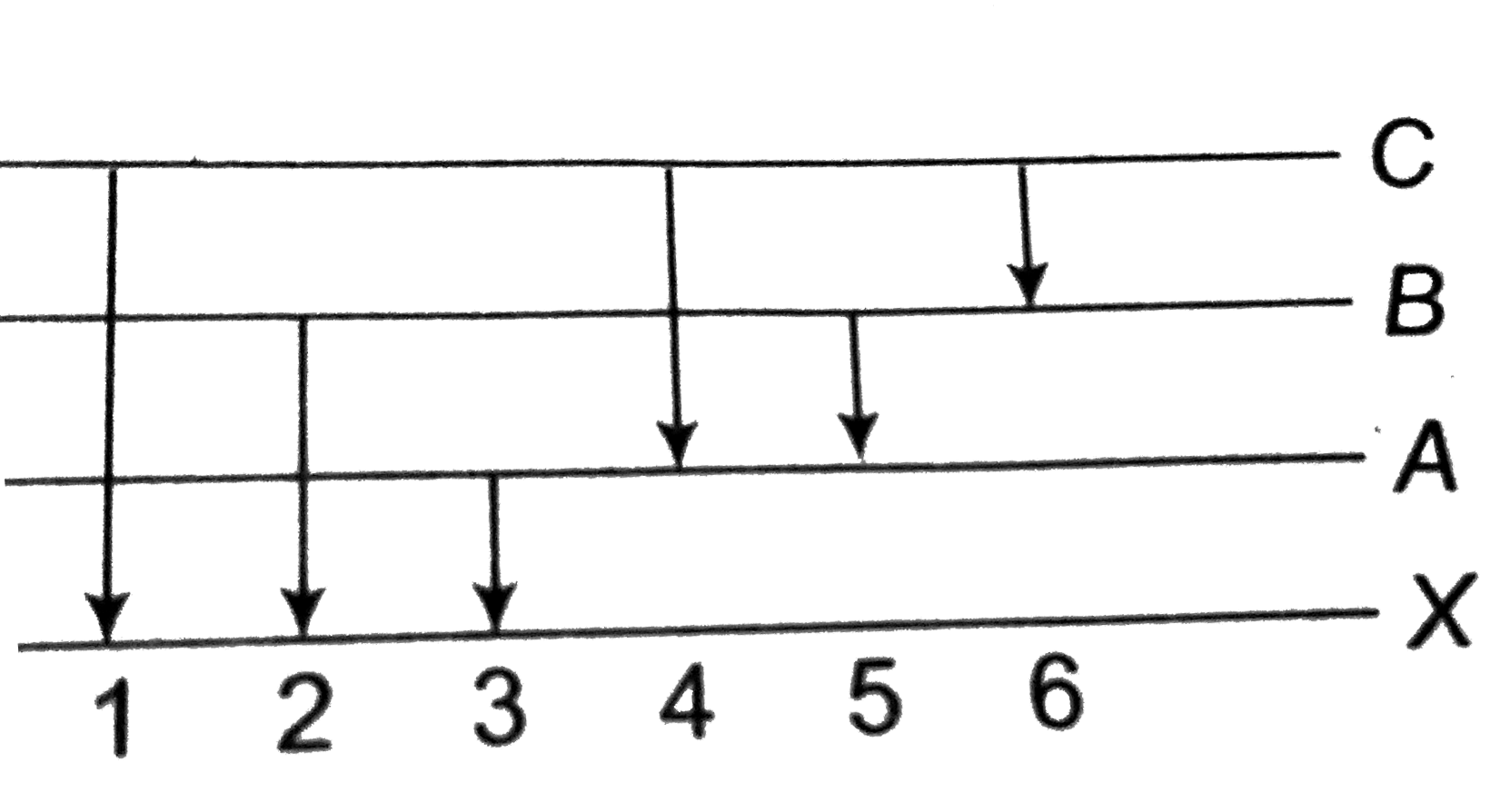
- Energy levels A, B, C of a certain atom corresponding to increasing va...
Text Solution
|
- Electrons in a certain energy level n=n(1) can emit 3 spectral lines. ...
Text Solution
|
- The figure indicates the enegry level diagram of an atom and the origi...
Text Solution
|
- The ionisation potential of hydrogen atom is -13.6 eV. An electron in...
Text Solution
|
- Hydrogen atom in ground state is excited by a monochromatic radiation ...
Text Solution
|
- A hydrogen sample is prepared in a particular state A photon of energy...
Text Solution
|
- A hydrogen atom in a state having a binding energy of 0.85 eV makes tr...
Text Solution
|
- Imagine an atom made of a proton and a hypothetical particle of double...
Text Solution
|
- In an excited state of hydrogen like atom an electron has total energy...
Text Solution
|
- Light form a dicharge tube containing hydrogen atoms falls on the surf...
Text Solution
|
- The electron in the hydrogen atom jumps from excited state (n=3) to it...
Text Solution
|
- Three photons coming from excited atoms hydrogen sample are picked up ...
Text Solution
|
- In a laboratory experiment on emission from atomic hydrogen in a disch...
Text Solution
|
- The de-Broglie wavelength of an electron in the first Bohr orbit is
Text Solution
|
- Consider an eelctron in the nth orbit of a hydrogen atom in the Bohr m...
Text Solution
|
- The ratio of the longest and shortest wavelengths of the Lyman series ...
Text Solution
|
- The ratio of longest wavelength and the shortest wavelength observed i...
Text Solution
|
- In terms of Rydberg's constant R, the wave number of the first Balman ...
Text Solution
|
- The wavelength of the first line of the Balmer series of hydrogen atom...
Text Solution
|
- The wavelength of the first line of Lyman series for hydrogen atom is ...
Text Solution
|