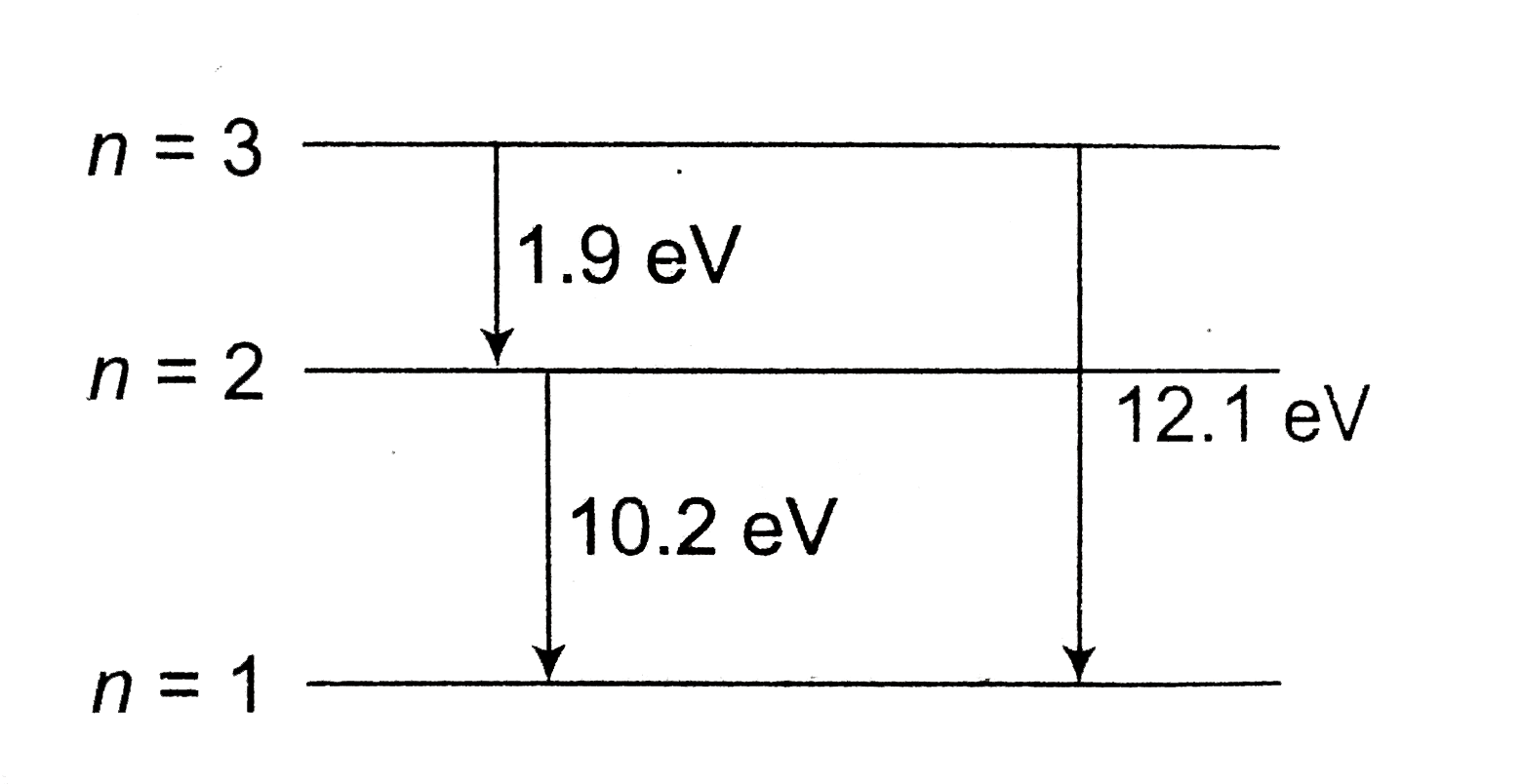A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CP SINGH-BOHR THEORY-Exercises
- Light form a dicharge tube containing hydrogen atoms falls on the surf...
Text Solution
|
- The electron in the hydrogen atom jumps from excited state (n=3) to it...
Text Solution
|
- Three photons coming from excited atoms hydrogen sample are picked up ...
Text Solution
|
- In a laboratory experiment on emission from atomic hydrogen in a disch...
Text Solution
|
- The de-Broglie wavelength of an electron in the first Bohr orbit is
Text Solution
|
- Consider an eelctron in the nth orbit of a hydrogen atom in the Bohr m...
Text Solution
|
- The ratio of the longest and shortest wavelengths of the Lyman series ...
Text Solution
|
- The ratio of longest wavelength and the shortest wavelength observed i...
Text Solution
|
- In terms of Rydberg's constant R, the wave number of the first Balman ...
Text Solution
|
- The wavelength of the first line of the Balmer series of hydrogen atom...
Text Solution
|
- The wavelength of the first line of Lyman series for hydrogen atom is ...
Text Solution
|
- Every series of hydrogen spectrum has an upper and lower limit in wave...
Text Solution
|
- Let v(1) be the frequency of series limit of Lyman series, v(2) the fr...
Text Solution
|
- The transtion from the state n = 4 to n = 3 in a hydrogen like atom r...
Text Solution
|
- Whenever a hydrogen atom emits a photon in the Balmer series, it may...
Text Solution
|
- A beam of ultraviolet light of all wavelength passes through hydrogen ...
Text Solution
|
- When a hydrogen atom emits a photon in going from n=5 to n=1, its reco...
Text Solution
|
- The radius of the hydrogen atom in its ground state is 5.3xx10^(-11) m...
Text Solution
|
- A photon of energy 10.2 eV corresponds to light of wavelength lamda(0...
Text Solution
|
- An electron with kinetic energy =E eV collides with a hydrogen atom in...
Text Solution
|