A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
FIITJEE-TEST PAPERS-CHEMISTRY
- How may molecules of RMgX are consumed in the above reaction.
Text Solution
|
- Amongst the following, total no of aromatic compounds are
Text Solution
|
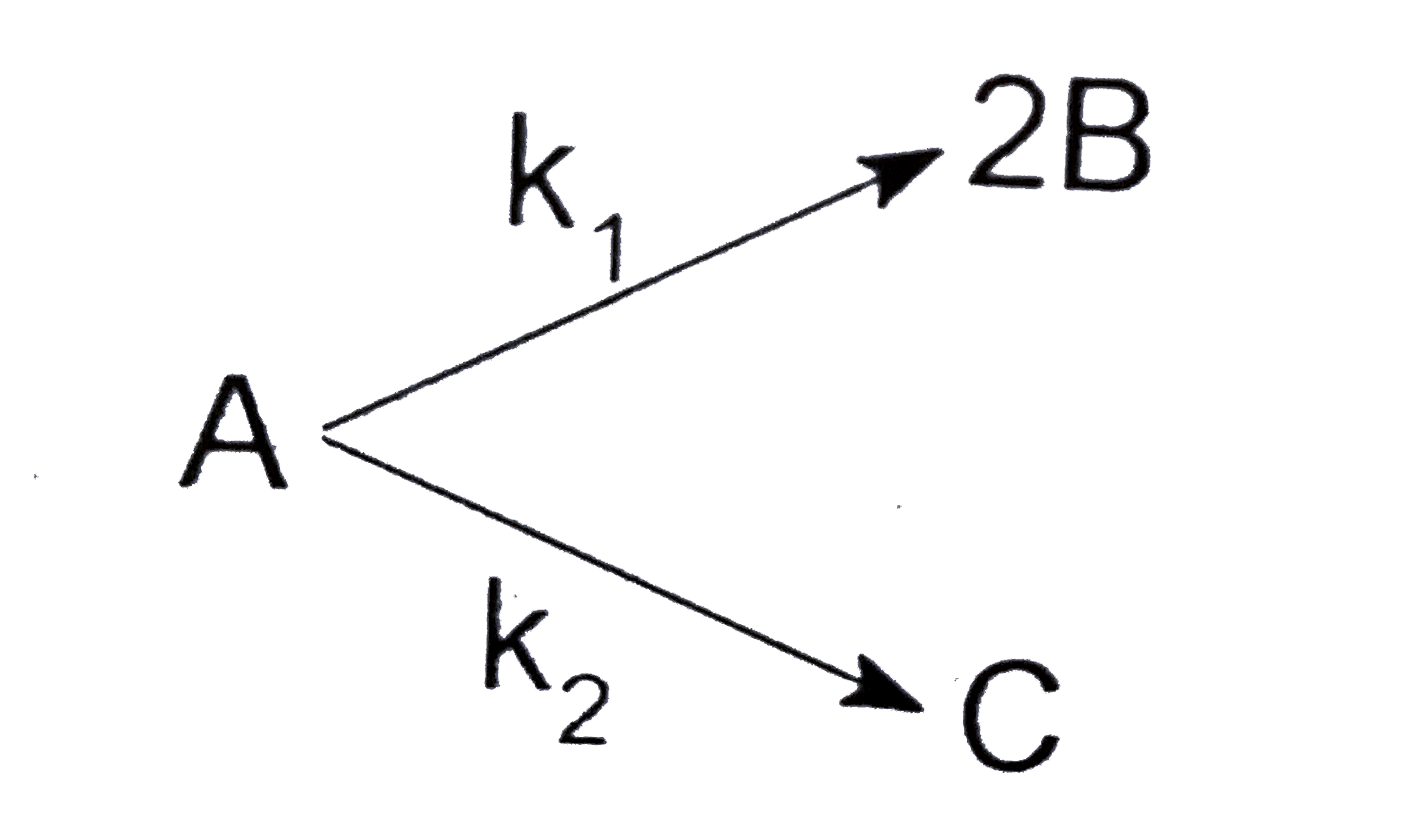
- For the following elementary first order reaction: If k(2)=2k(1),...
Text Solution
|
- One of the allotropic form of boron is alpha - rhombohedral boron. Num...
Text Solution
|
- Which of the folowing is bio-degradable polymer?
Text Solution
|
- White phosphorus is a tetra atomic solid P(4) (s) at room temperature....
Text Solution
|
- In the exteraction of copper from its sulphide ore, the metal is fanal...
Text Solution
|
- Order of basic nature is A gt B gt C. Order of % of A, B and C in ov...
Text Solution
|
- A cell contain two hydrogen electrodes. The negatove electrode is in c...
Text Solution
|
- The image on an exposed and developed photographic film is due to
Text Solution
|
- Wood spirit is obtained by catalytic reaction of water gas with hydrog...
Text Solution
|
- The compressibility factor (Z) of real gas is usually less than 1 at l...
Text Solution
|
- Zn+conc.HNO(3) rarr Zn(NO(3))(2)+X+H(2)O Zn+dil.HNO(3) rarr Zn(NO(3)...
Text Solution
|
- Which of the following compound will give blood red colour while doing...
Text Solution
|
- On mixing which of the following solutions, observed boiling point of ...
Text Solution
|
- The E.N. of H,X,O are 2.1, 0.8 and 3.5 respectively comment on the nat...
Text Solution
|
- Acetylene on passing through excess of HOCl solution forms
Text Solution
|
- 50 ml, 0.1M NaHCO(3) aqueous solution is mixed with 50 ml, 0.8 M Na(2)...
Text Solution
|
- In which of the following compexes of Co, the magnitude of CFSE (Delt...
Text Solution
|
- In simplified version of nucleic acid chain, nucleotides are joined to...
Text Solution
|