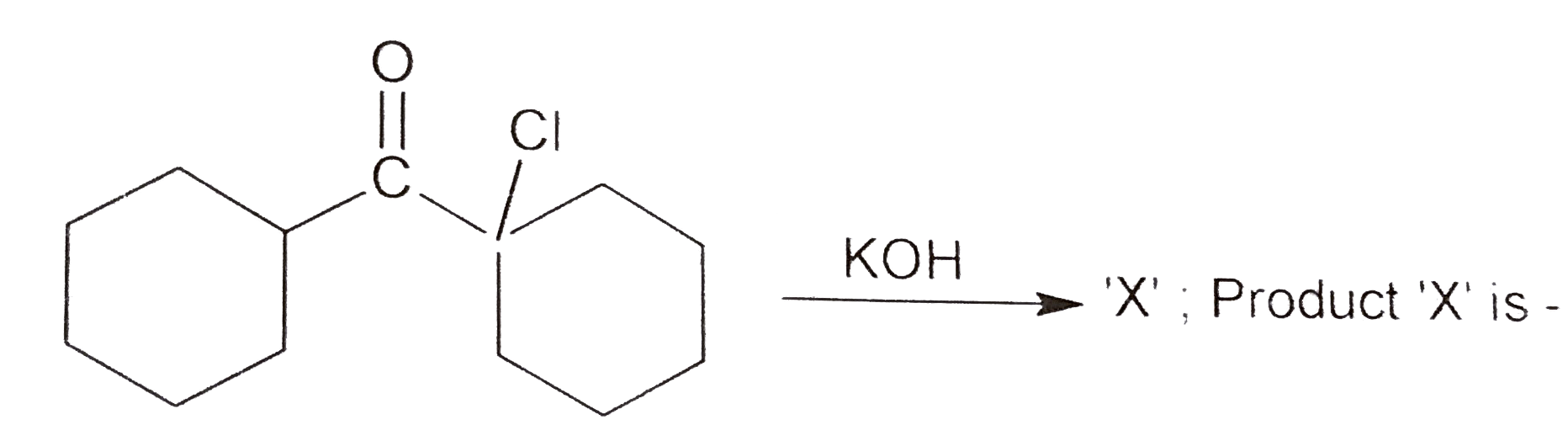
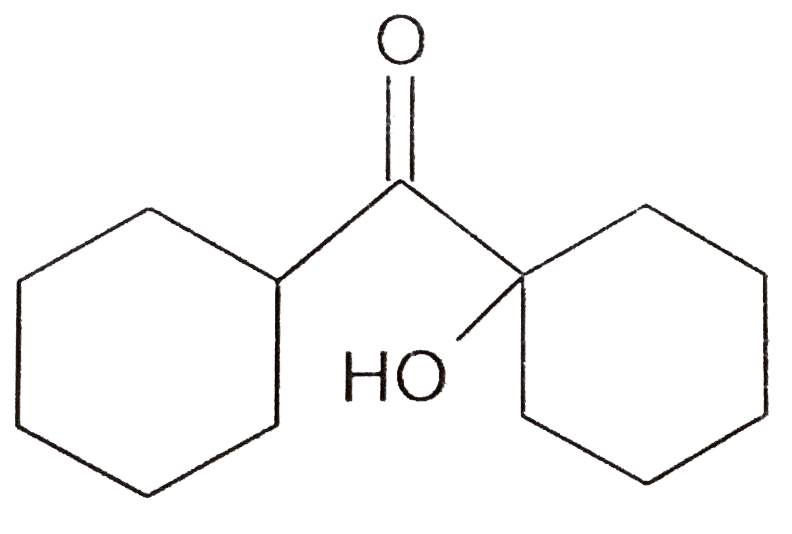
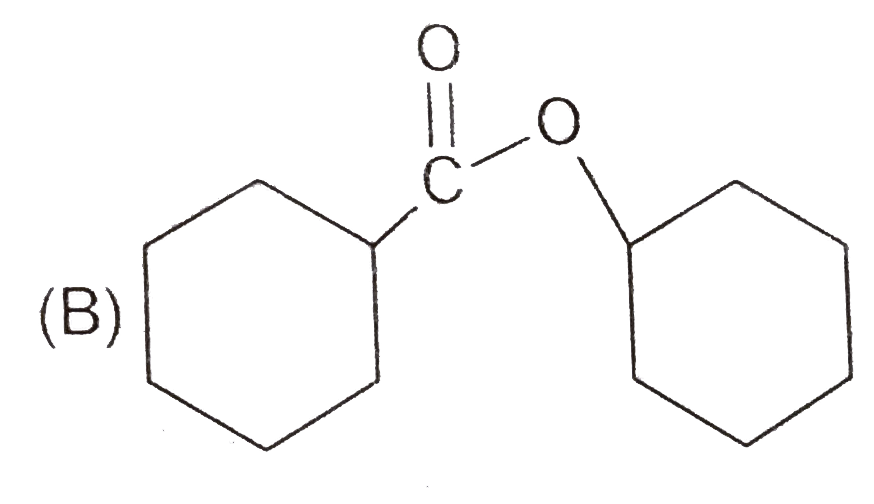
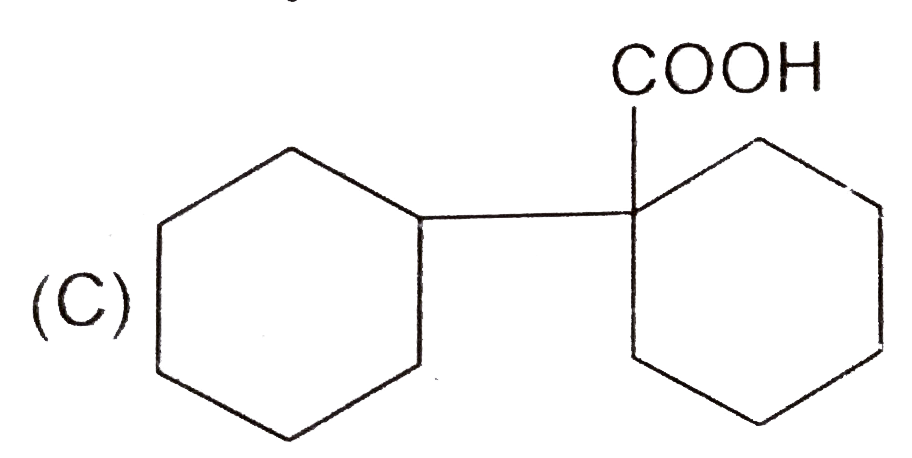
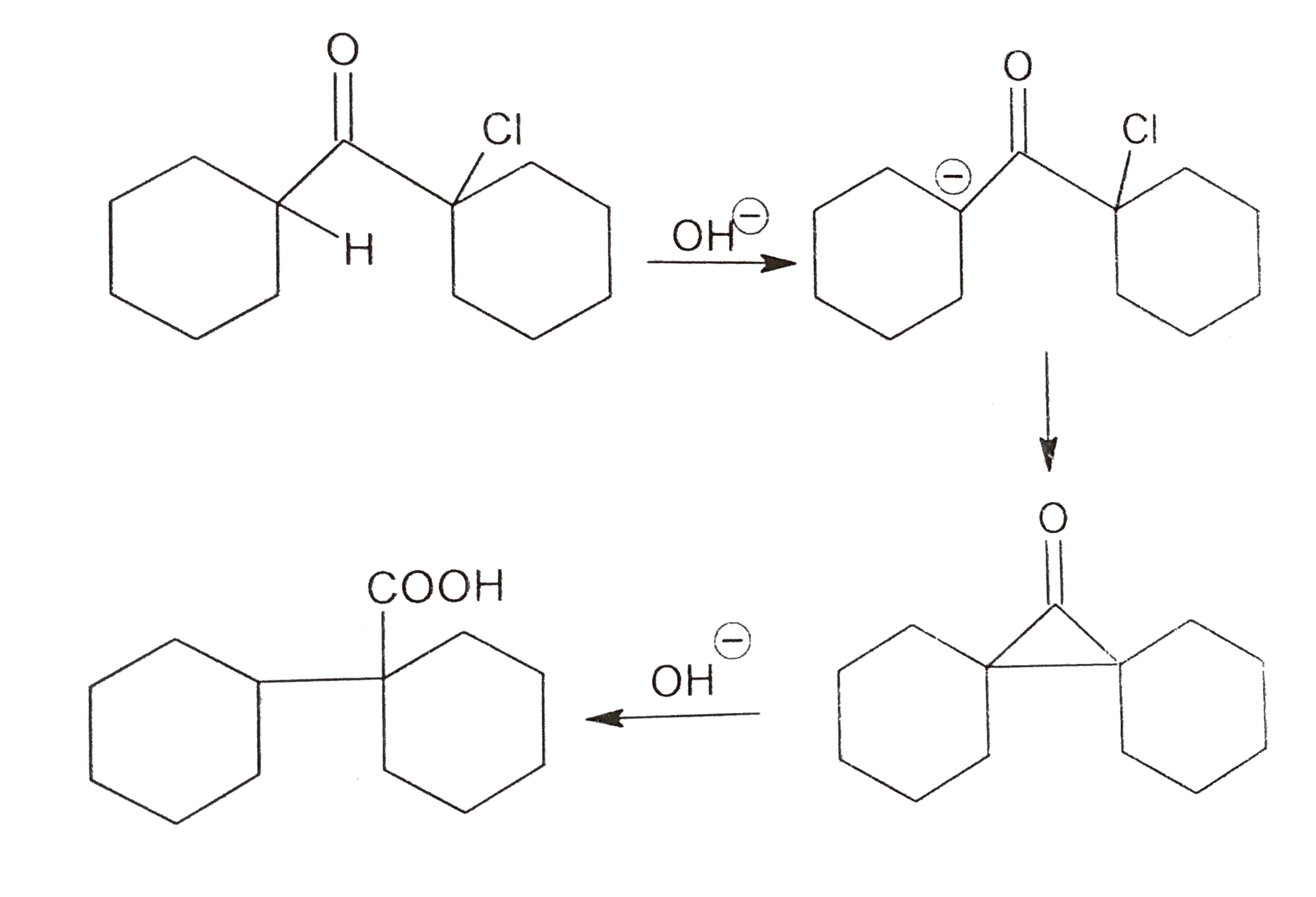
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
FIITJEE-TEST PAPERS-CHEMISTRY
- Complete the following reaction
Text Solution
|
- A and B in the above reactions will be:
Text Solution
|
- Complete the following reaction
Text Solution
|
- Choose the incorrect statements/s among the following: (1) There ar...
Text Solution
|
- 1 g charcoal is placed in 100 mL of 0.5 M CH(3)COOH to form an adsorbe...
Text Solution
|
- {:(Na(2)B(4)O(7),+,H(2)SO(4),rarr,A,overset(H(2)O)rarr,,),(,,,,,,darr,...
Text Solution
|
- The energy produced by the de-excitation of (1)/(1000) moles of H - at...
Text Solution
|
- The composition of sample is F(0.93)O(1.00). Find the correct code for...
Text Solution
|
- A 0.1 M solution of a weak acid HA is titrated by NaOH slowly. The ti...
Text Solution
|
- , The true statement about product 'X' will be:
Text Solution
|
- The position of equilibrium lies to the right in each of these reactio...
Text Solution
|
- Considering no electronic repulsion in Helium atom what will be Bohr's...
Text Solution
|
- Choose the correct option regarding d(p - o) (single bond length)
Text Solution
|
- Ph-underset(underset(Cl)(|))(CH)-CH(2)-Cl underset(underset(("iii") H^...
Text Solution
|
- , The product 'A' will be
Text Solution
|
- An aqueous solution has a density of 1. 37 g//ml. If molecular wt of s...
Text Solution
|
- Calculate the degree og ionisation of 0.04 M HOCl solution having ioni...
Text Solution
|
- In a crystalline solid, X atoms occupy hcp and 1//4 th of tetrahedral ...
Text Solution
|
- Bohr's model is able to explain
Text Solution
|
- On icreasing temperature from 200 K to 220 K rate of reaction A increa...
Text Solution
|