Similar Questions
Explore conceptually related problems
Recommended Questions
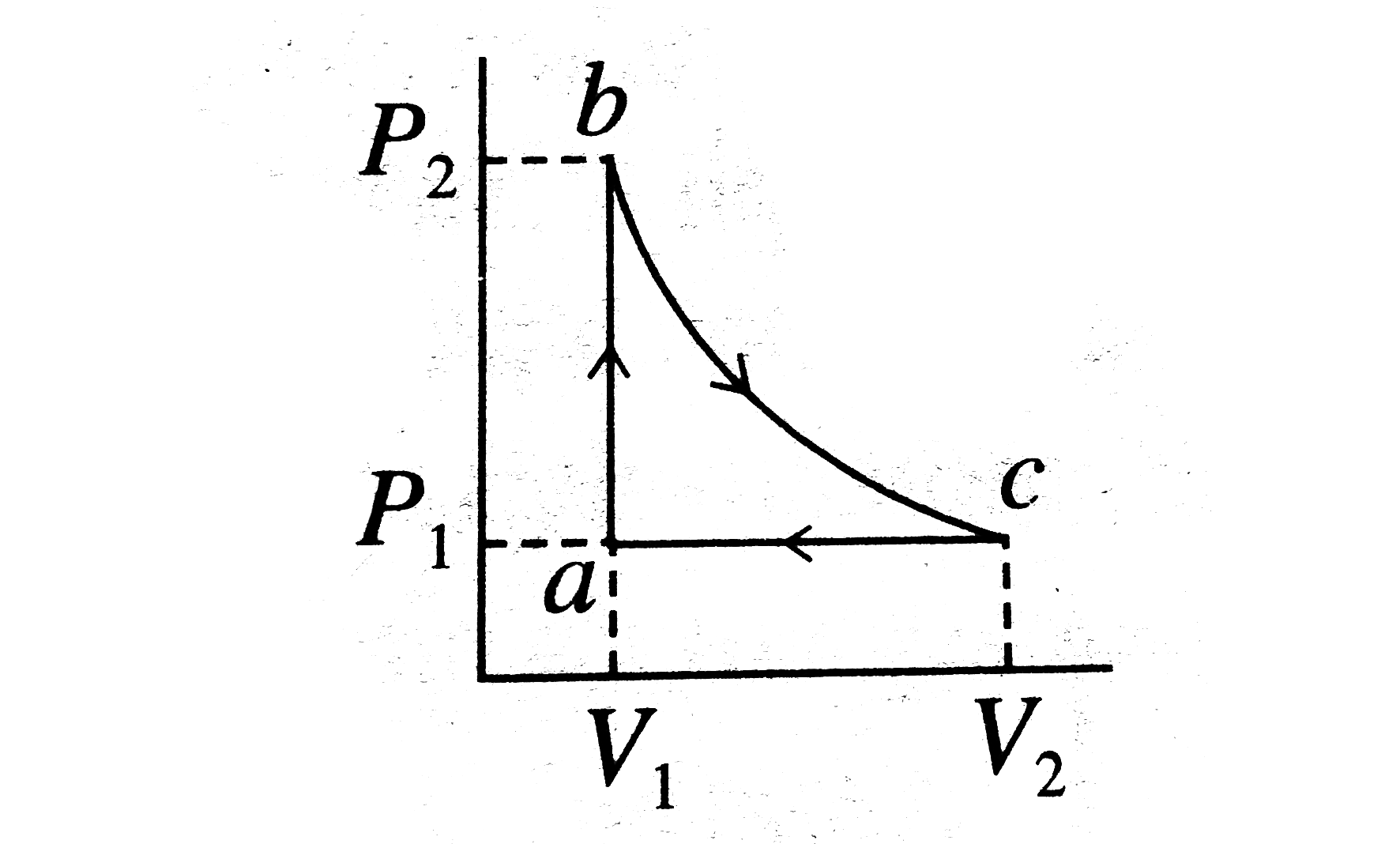
- Carbon monoxide is carried around a closed cyclic processes abc, in wh...
Text Solution
|
- Carbon monoxide is carried around a closed cyclic processes abc, in wh...
Text Solution
|
- In a cyclic process shown in the figure an ideal gas is adial gas is a...
Text Solution
|
- Consider the following cyclic abcda performed on 2 mole of an ideal ga...
Text Solution
|
- In the figure n mole of a monoatomic ideal gas undergo the process ABC...
Text Solution
|
- An ideal gas follows a cyclic process as shown in figure. Internal ene...
Text Solution
|
- One mole of an ideal monoatomic gas undergoes a cyclic process, as sho...
Text Solution
|
- Figure shows the variation of internal energy (U) with the pressure (P...
Text Solution
|
- consider the cyclic process ABCA on a sample of 2.0 mol of an ideal ga...
Text Solution
|