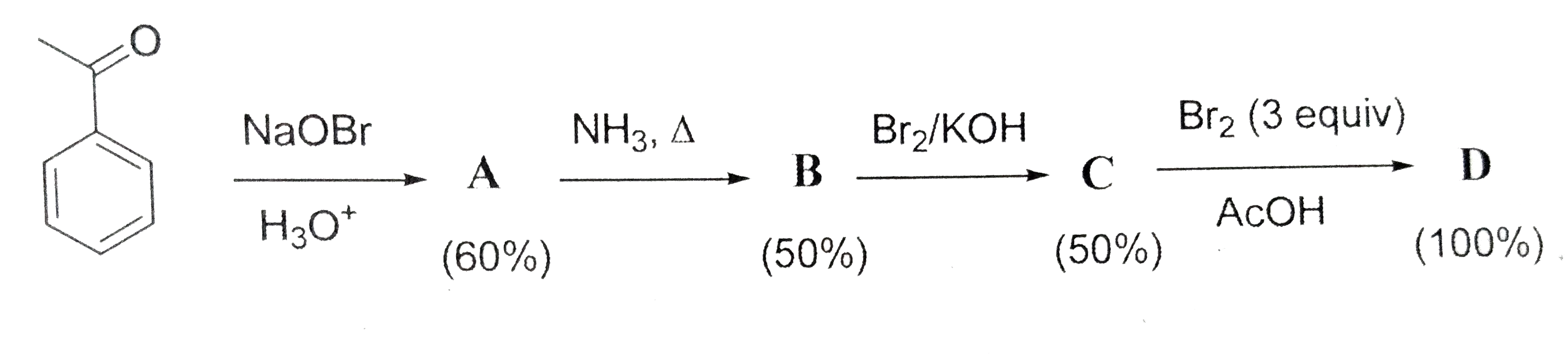Similar Questions
Explore conceptually related problems
Recommended Questions
- In the following reaction sequence, the amount of D (in g) formed from...
Text Solution
|
- Statement 1 "mole" O(3)=N "molecule"O(3)=3N atoms of O=48 g Explanat...
Text Solution
|
- 0.05 mole of LiAlH(4) in ether solution was placed in a flask containi...
Text Solution
|
- In the following reaction sequence, the amount of D (in g) formed from...
Text Solution
|
- In the following reaction sequence, the amount of D (in g) formed from...
Text Solution
|
- In the following reaction sequence, the amount of D (in g) formed form...
Text Solution
|
- Consider the following reactions. Molecular weight of the product woul...
Text Solution
|
- In the following sequence of reactions, the amount of D (in g) formed ...
Text Solution
|
- What is the percentage of carbon in urea? (Atomic mass C = 12, H = 1, ...
Text Solution
|