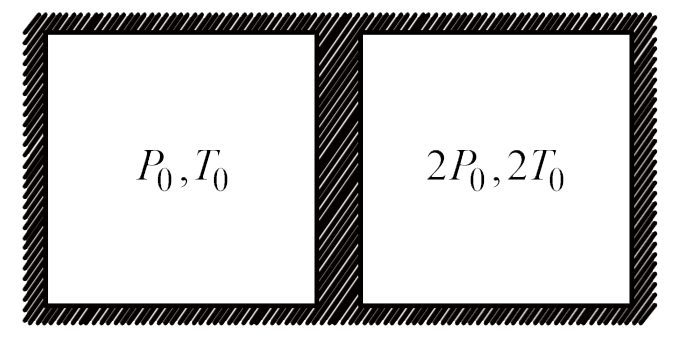Recommended Questions
- A diathermic piston divides adiabatic cylinder of volume V0 into two e...
Text Solution
|
- Figure shows an insulated cylinder of volume V containing monatomic ga...
Text Solution
|
- Figure shows an insulated cylinder of volume V containing monatomic ga...
Text Solution
|
- Figure shows an insulated cylinder of volume V containing monatomic g...
Text Solution
|
- Figure shows an insulated cylinder of volume V containing monatomic g...
Text Solution
|
- Figure shows an insulated cylinder of volume V containing monatomic g...
Text Solution
|
- A diathermic piston divides adiabatic cylinder of volume V0 into two e...
Text Solution
|
- A diathermic piston divides adiabatic cylinder of volume V0 into two e...
Text Solution
|
- A diathermic piston divides adiabatic cylinder of volume V0 into two e...
Text Solution
|