A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-ATOMIC PHYSICS-Exercise
- If the short wavelength limit of the continous spectrum coming out of ...
Text Solution
|
- A particle of charge q(0) and of mass m(0) is projected along the y-ax...
Text Solution
|
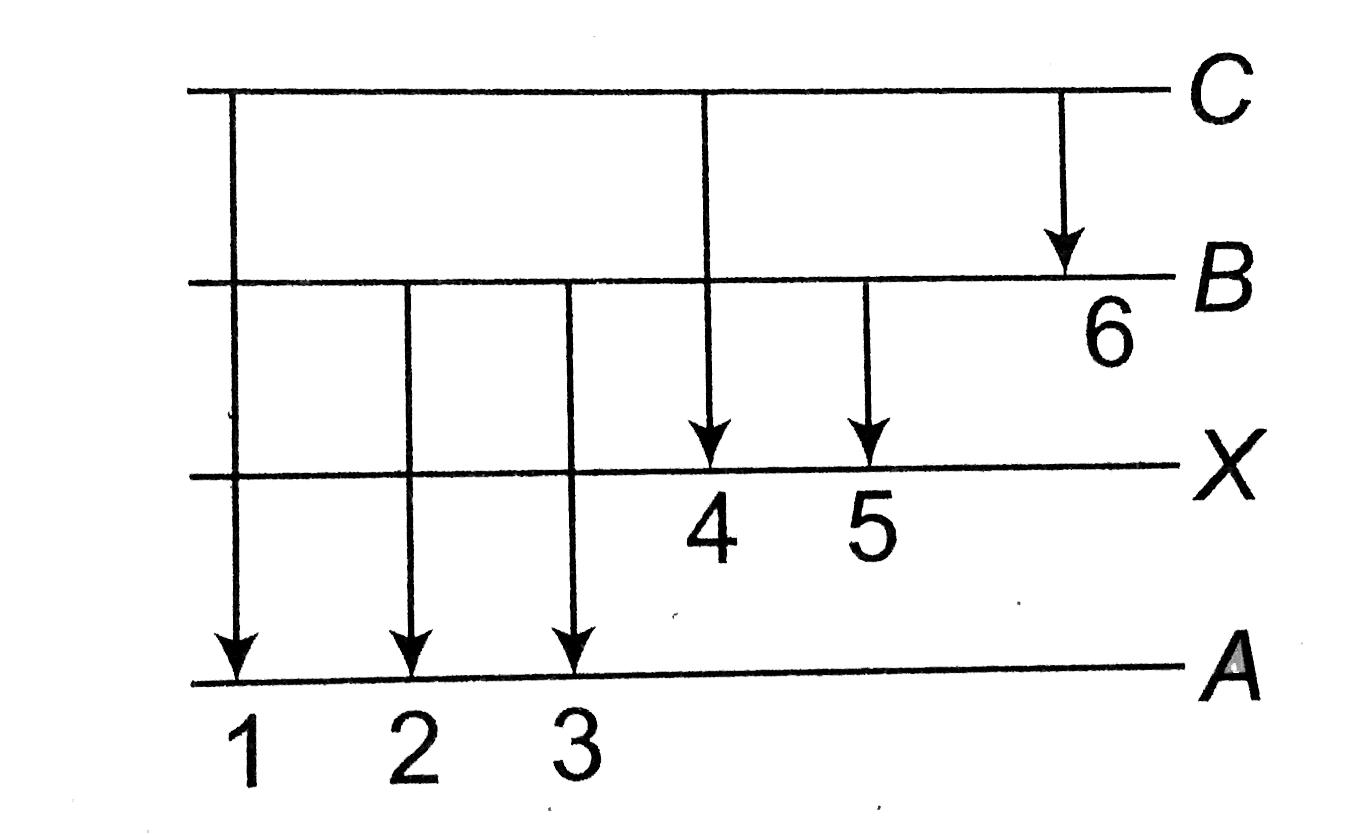
- In the figure six lines of emission spectrum are shown. Which of them ...
Text Solution
|
- Consider atoms H, He^(+), Li^(++) in their ground states. If L(1), L(2...
Text Solution
|
- In the photoelectric experiment, if we use a monochromatic light, the ...
Text Solution
|
- Which of the following is incorrect statement regarding photon
Text Solution
|
- A monochromatic beam of electromagnetic radiation has an intensity of ...
Text Solution
|
- Yellow light of 557 nm wavelength is incident on a cesium surface. It ...
Text Solution
|
- A point source causes photoelectric effect from a small metal plate. W...
Text Solution
|
- Let (nr) and (nb) be respectively the number of photons emitted by a r...
Text Solution
|
- When light of intensity 1W//m^2 and wavelength 5xx10^(-7)m is incident...
Text Solution
|
- If we go on increasing the wavelength of light incident on a metal sur...
Text Solution
|
- The energy of a photon is E = hv and the momentum of photon p = (h)/(l...
Text Solution
|
- Light of wavelength 5000 Å falls on a sensitive plate with photoelectr...
Text Solution
|
- The photoelectric work function for a metal surface is 4.125 eV. The c...
Text Solution
|
- The wavelength of a photon and the deBroglie wavelength of an electron...
Text Solution
|
- A proton and an alpha - particle are accelerated through same potentia...
Text Solution
|
- Electron microscope works on the principle of
Text Solution
|
- What voltage must be applied to an electron microscope to produce elec...
Text Solution
|
- Linear momenta of a proton and an electron are equal. Relative to an e...
Text Solution
|