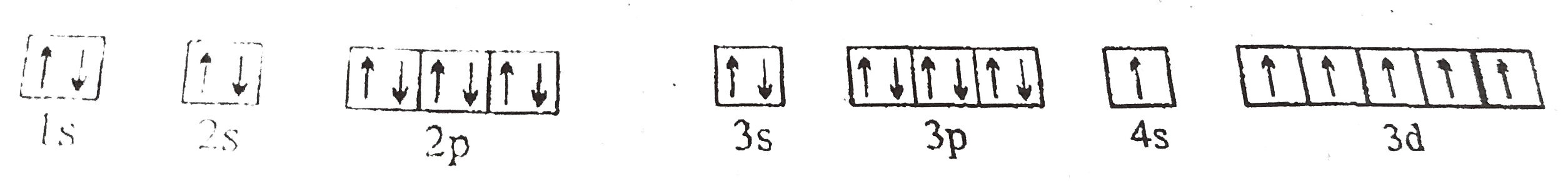
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-ATOMIC STRUCTURE-PHYSICAL CHEMISTRY(ATOMIC EQUILIBRIUM)
- The number of possible lines of Paschenc series when electron jumps fr...
Text Solution
|
- AIR service on Vividh Bharati is transmitted on 219m band. What is its...
Text Solution
|
- Calculate the maximum and minimum number of electrons. Which may have ...
Text Solution
|
- In the emission spectrum of H- atom from energy level 'n' to ground s...
Text Solution
|
- The ionisation energy of H is 13.6 eV. Calculate the ionization energy...
Text Solution
|
- If Photon having wavelength 6.2nmwas allowed to strike a metal plate h...
Text Solution
|
- The number of nodal planes in a p(x) orbital is :
Text Solution
|
- The wave number of electromagnetic radiation emitted during the trans...
Text Solution
|
- The correct order of wavelength of Hydrogen (.(1)H^(1)), Deuterium (.(...
Text Solution
|
- Spin angular momentum for unpaired electron in sodium ( Atomic No. =11...
Text Solution
|
- The distance between 4th and 3rd Bohr orbits of He^(+) is :
Text Solution
|
- What is the frequency of revolution of electron present in 2nd Bohr's ...
Text Solution
|
- According to Bohr's atomic theory, which of the following is correct ?
Text Solution
|
- If in Bohr's model, for unielectronic atom, time period of revolution ...
Text Solution
|
- Be^(3+) and a proton are accelerated by the same potential, their de-...
Text Solution
|
- If the ionization energy of He^(+) is 19.6xx10^(-18) J per atom then t...
Text Solution
|
- The mass of a particle is 10^(-10)g and its radius is 2xx10^(-4)cm. If...
Text Solution
|
- What is the shortest wavelength line in the Paschen series of Li^(2+) ...
Text Solution
|
- A dye absorbs a photon of wavelength lambda and re- emits the same ene...
Text Solution
|
- A hydrogen atom in the ground state is excited by monochromatic radia...
Text Solution
|