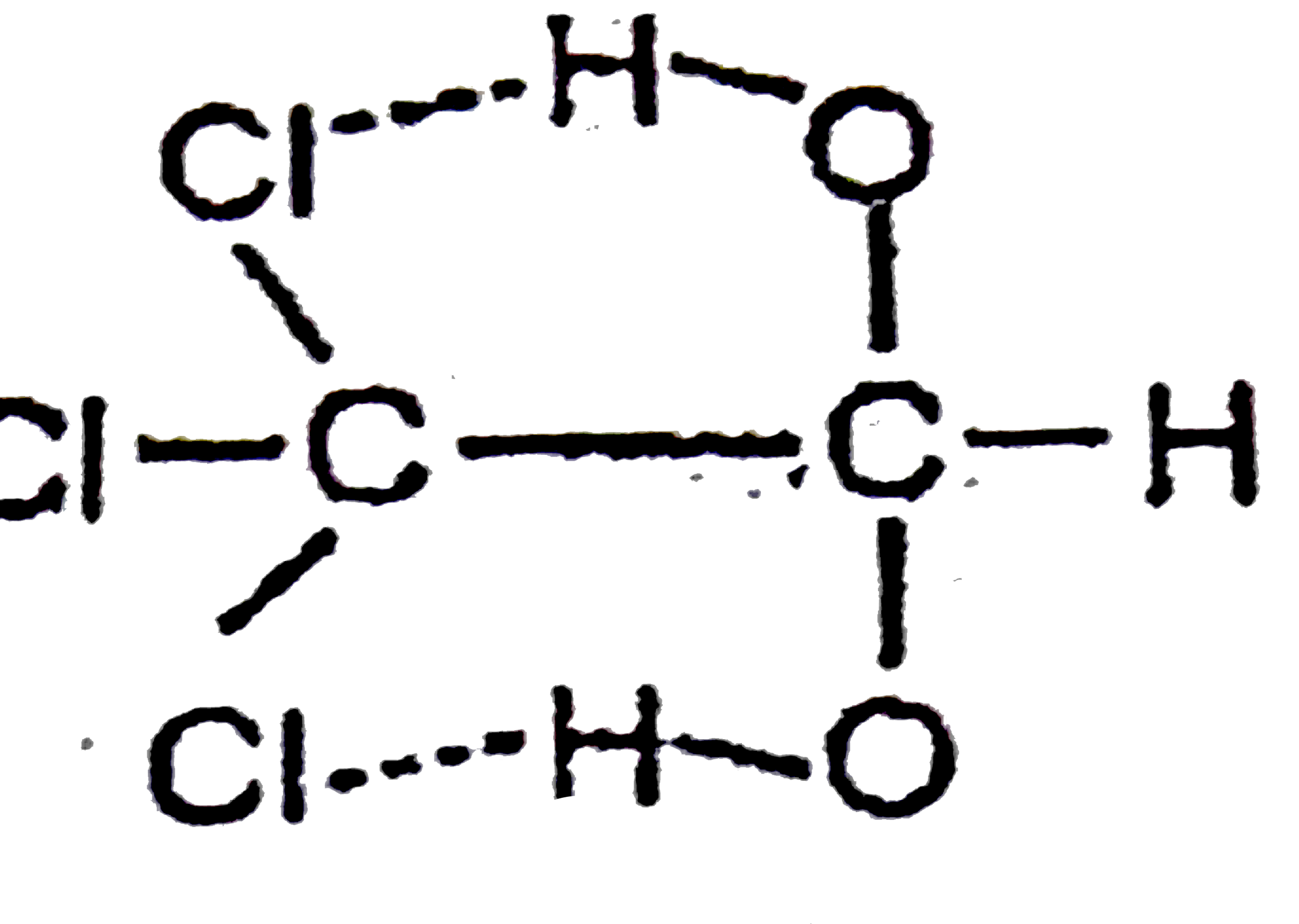
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-ATOMIC STRUCTURE-PHYSICAL CHEMISTRY(ATOMIC EQUILIBRIUM)
- According to Bohr's atomic theory, which of the following is correct ?
Text Solution
|
- If in Bohr's model, for unielectronic atom, time period of revolution ...
Text Solution
|
- Be^(3+) and a proton are accelerated by the same potential, their de-...
Text Solution
|
- If the ionization energy of He^(+) is 19.6xx10^(-18) J per atom then t...
Text Solution
|
- The mass of a particle is 10^(-10)g and its radius is 2xx10^(-4)cm. If...
Text Solution
|
- What is the shortest wavelength line in the Paschen series of Li^(2+) ...
Text Solution
|
- A dye absorbs a photon of wavelength lambda and re- emits the same ene...
Text Solution
|
- A hydrogen atom in the ground state is excited by monochromatic radia...
Text Solution
|
- The ratio of magnetic moments of Fe(III) and Co(II) is :
Text Solution
|
- A compound of vanadium has a magneitc moment (mu) of 1.73 BM. If the v...
Text Solution
|
- According to Bohr's theory, the ratio of electrostatic force of attrac...
Text Solution
|
- In a sample of H- atom electrons make transition from 5^(th) excited ...
Text Solution
|
- Number of electron having l+m value equal to zero in .(26)Fe may be
Text Solution
|
- 1^(st) excitation Potential of a hydrogen like sample is 15 volt. If ...
Text Solution
|
- The ratio of the wave number corresponding to the 1^(st) line of Lyma...
Text Solution
|
- A particle X moving with a certain velocity has a debragile wave lengt...
Text Solution
|
- Photon having wavelength 310nm is used to break the bond of A(2) molec...
Text Solution
|
- If the wavelength of photon emitted from an electron jump n=4 to n=2 i...
Text Solution
|
- Electron in a sample of H- atoms make transitions from state n=x to so...
Text Solution
|
- Infrared lamps are used in restaurants to keep the food warm. The infr...
Text Solution
|