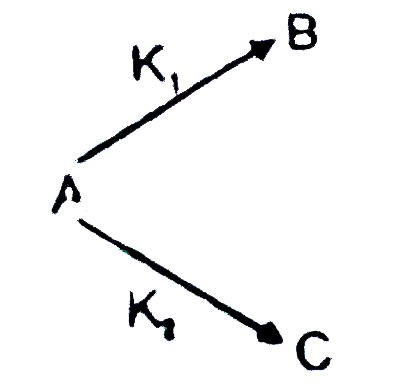
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-CHEMICAL KINETICS-PHYSICAL CHEMITRY (CHEMICAL KNIETICS & RADIOACTIVITY)
- Half life of reaction : H(2)O(2)(aq) rarr H(2)O(l)+(1)/(2)O(2)(g)is in...
Text Solution
|
- A first - order reaction is 20% complete in 10 minutes. Calculate the ...
Text Solution
|
- A subtance undergoes first order decomposition involving two parallel ...
Text Solution
|
- A reaction A(2)+B rarr products, involves the following mechanism : ...
Text Solution
|
- In a reaction A rarr products , when start is made from 8.0 xx 10^(-2...
Text Solution
|
- A catalyst lowers the enrgy of activation by 25%, temperature at which...
Text Solution
|
- Half life of a first order reaction is 69.3 minutes. Time required to ...
Text Solution
|
- Gasesous cyclobutane isomerizes to butadiene following first order pro...
Text Solution
|
- For a first order for the reaction A rarr products : the rate of reac...
Text Solution
|
- The rate constant for the reaction 2N(2)O(5)rarr 4NO(3)+O(2) is 3.0 xx...
Text Solution
|
- The decomposition of N(2)O into N(2) and O(2) in presence of gaseous a...
Text Solution
|
- The rate of a heterogeneous reaction (as iron (solid) any oxygen gas) ...
Text Solution
|
- For a chemical reaction A rarr Products, the rate of disappearance of ...
Text Solution
|
- At the point of intersection of the two curves shown, the conc. of B i...
Text Solution
|
- The decomposition of N(2)O(5) occurs as, 2N(2)O(5) rarr4NO(2)+O(2) and...
Text Solution
|
- The rate constant for an isomerization reaction, A rarr B is 4.5 xx 10...
Text Solution
|
- A hypothetical reaction A(2) + B(2) rarr 2AB follows the mechanism as ...
Text Solution
|
- For an exothermic chemical process occurring in two as (i) A + B rar...
Text Solution
|
- For the reaction 2NO(2)+F(2)rarr 2NO(2)F, following mechanism has been...
Text Solution
|
- For the reaction, 2NO(g) + 2H(2)(g) rarr N(2)(g) + 2H(2)O(g) The ...
Text Solution
|