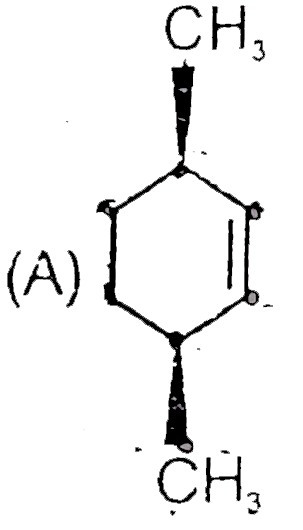
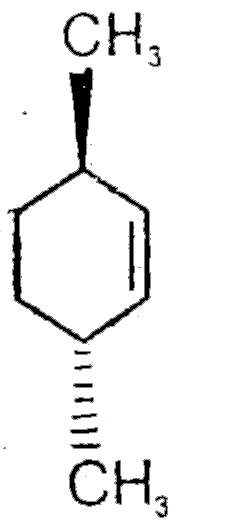
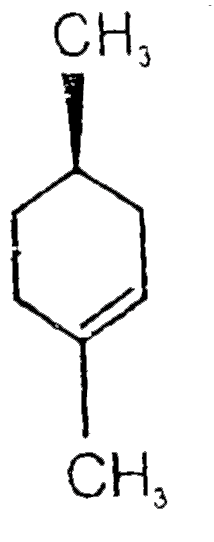
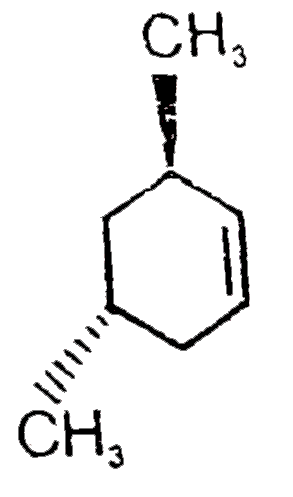
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- An optically active compound A with molecular formula C(6)H(14) underg...
Text Solution
|
- Give the structure of an optically active alkene (A) having the lowest...
Text Solution
|
- An optically active hydrocarbon X has molecular formula C(6)H(12) . X ...
Text Solution
|
- An optically active compound A with molecular formula C(6)H(14) underg...
Text Solution
|
- An optically active compound A with molecular formula C(8)H(14) underg...
Text Solution
|
- C(C(6)H(12)) , and optically active dydrocarbon which on catalytic hyd...
Text Solution
|
- C(C(6)H(12)), and optically active dydrocarbon which on catalytic hydr...
Text Solution
|
- Optically active 'P' has the molecular formula C(6) H(12) and catalyti...
Text Solution
|
- एक धुवण घूर्णक (Optically Active) हाइड्रोकार्बन (D) C(6)H(12)जो उत्प्र...
Text Solution
|