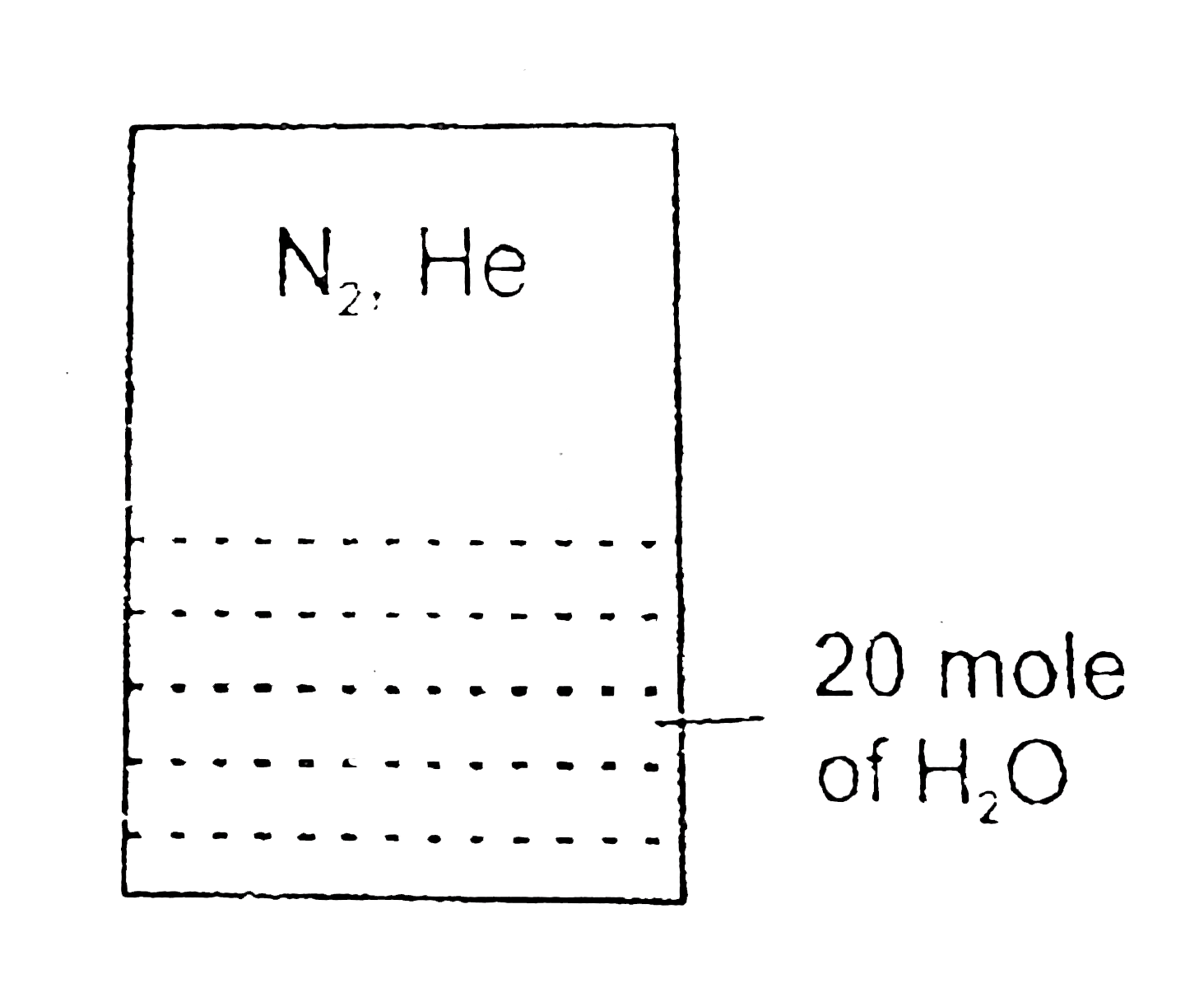Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In the arrangement given below 20 mole of N(2) and 5 mole of He are pr...
Text Solution
|
- Henry 's law constant for the solubility of N(2) gas in water at 298 K...
Text Solution
|
- The Henry's law constant for the solubility of N(2) gas in water at 29...
Text Solution
|
- The Henry's law constant for the solubility of N(2) gas in water at 29...
Text Solution
|
- In the arrangement given below 20 mole of N(2) and 5 mole of He are pr...
Text Solution
|
- The Henry's law constant for the solubility of N(2) gas i water at 298...
Text Solution
|
- The Henry's law constant fo the solubility of N(2) gas in water at 29...
Text Solution
|
- The Henry's law constant for the solubility of N(2) gas in water at 29...
Text Solution
|
- The Henry's law constant for the solubility of N(2) gas in water at 29...
Text Solution
|