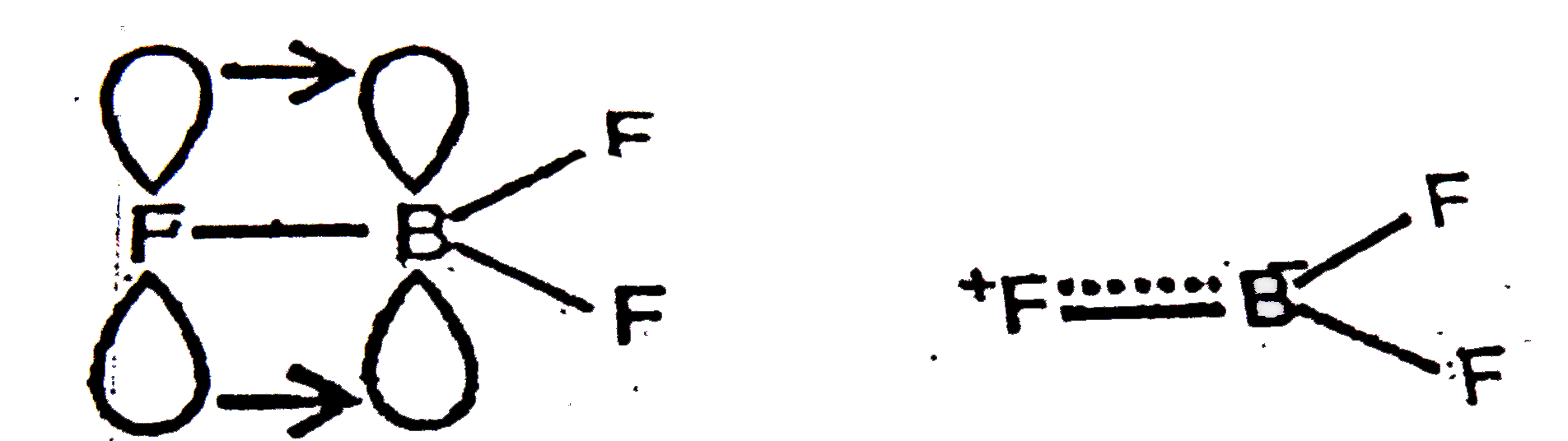A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P BLOCK ELEMENTS
RESONANCE|Exercise Example|30 VideosP BLOCK ELEMENTS
RESONANCE|Exercise Exercise 1 part 1 subjective ques|30 VideosNUCLEAR CHEMISTRY
RESONANCE|Exercise STAGE-II|1 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE|Exercise PART - III : OLYMPIAD PROBLEMS (PREVIOUS YEARS) STAGE - V (INTERNATIONAL CHEMISTRY OLYMPIAD (IChO)) Problem 3|8 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-P BLOCK ELEMENTS-PART -II
- The Lewis acid character of boron trihalides decreases as: B Br(3) gt ...
Text Solution
|
- Write the two important minerals of fluorine.
Text Solution
|
- Name four oxyacids of chlorine. Give their molecular formulae.
Text Solution
|
- Name the oxide of chlorine which has odd number of electrons and param...
Text Solution
|
- What is the colour of vapours obtained when an iodide is heated with c...
Text Solution
|
- Freshly distilled colourless HI (aqueous solution) gradually turns bro...
Text Solution
|
- A certain compound (X) shows the following reactions. (i)When Kl is ...
Text Solution
|
- Write is quantum mechanical liquid i.e. helium (II) ? Give its two imp...
Text Solution
|
- Write down the formula of the noble gas species which are isostructura...
Text Solution
|
- Distillation of HBr and HBrO3 yields bromine gas.
Text Solution
|
- Pl3 on hydrolysis yields H3PO3 and HI
Text Solution
|
- IO(3)^(-) + 6OH^(-)+Cl(2) to IO(6)^(5-) ("periodate")+3H2O+2Cl^(-)
Text Solution
|
- HBr is a stronger acid than HI because of hydrogen bonding.
Text Solution
|
- Bleaching powder does not show oxidising properties.
Text Solution
|
- Noble gases are parmagnetic in nature.
Text Solution
|
- Neon lights are visible even in fog and moist.
Text Solution
|
- The high activity of fluorine is due to its …. Dissociation energy
Text Solution
|
- The atomicity of all halogens is ….
Text Solution
|
- Among halogen acids (hydrogen halides) ….. Is the strongest reducing a...
Text Solution
|
- K2Cr2O7+HCl to KCl+CrCl3++
Text Solution
|
- Iodine reacts with hot concentrated NaOH solution. The products are Na...
Text Solution
|