Similar Questions
Explore conceptually related problems
Recommended Questions
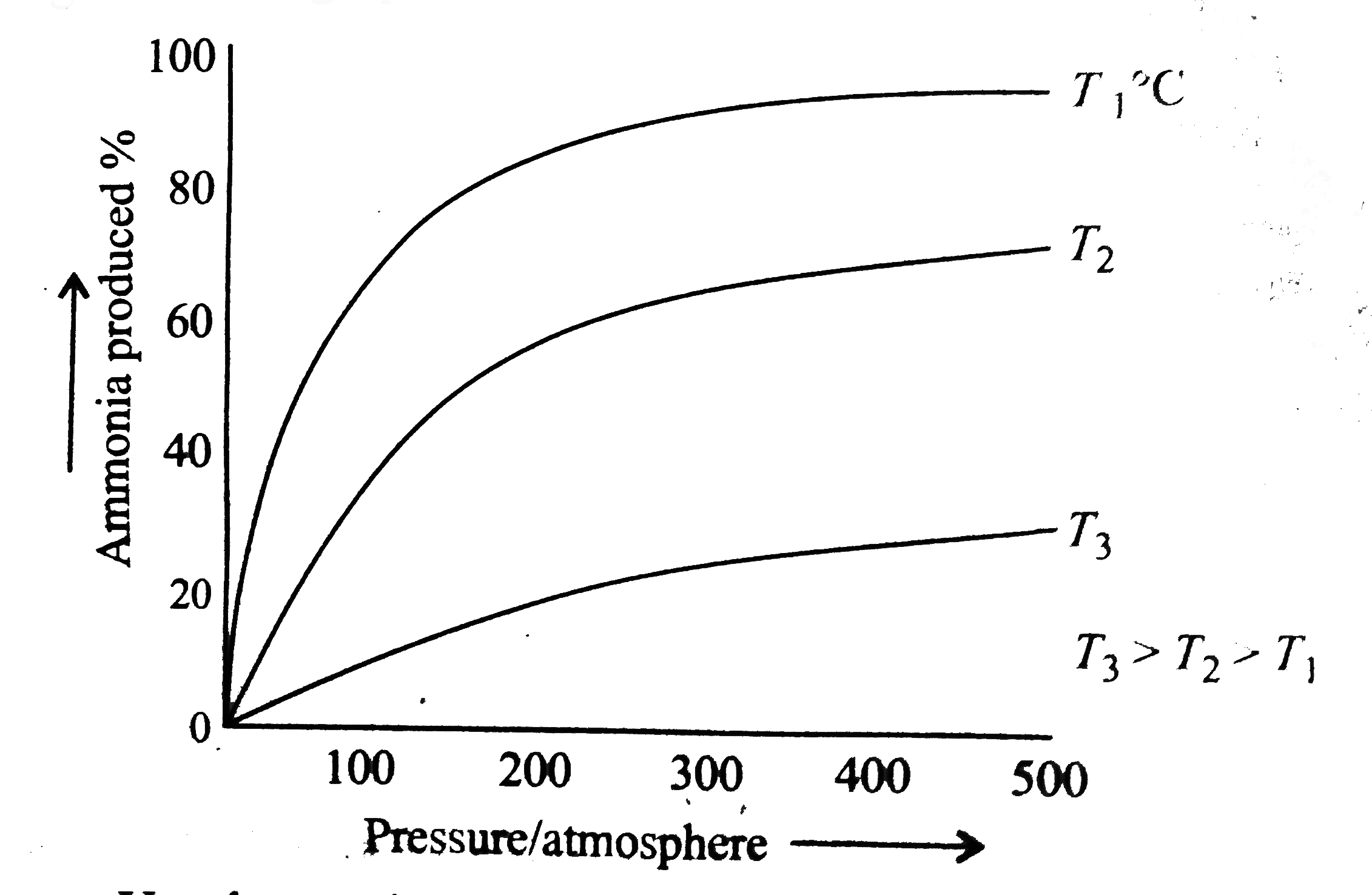
- The persentage of ammonia produced from nitrogen and hydrogen under co...
Text Solution
|
- The persentage of ammonia produced from nitrogen and hydrogen under co...
Text Solution
|
- The persentage of ammonia produced from nitrogen and hydrogen under co...
Text Solution
|
- The persentage of ammonia produced from nitrogen and hydrogen under co...
Text Solution
|
- High pressure and low temperature are favourable conditions for the sy...
Text Solution
|
- Nitrogen and hydrogen combine to form ammonia under high temperature a...
Text Solution
|
- In the reaction, N(2)+ 3" H"(2) rarr 2 NH(3), the ratio of volumes o...
Text Solution
|
- Study the following graph carefully and answer the given questions. ...
Text Solution
|
- नाइट्रोजन एवं हाइड्रोजन उच्च ताप एवं दाब की स्थितियों में संयुक्त होकर...
Text Solution
|